Question: Can you show a step-by-step process on how to solve this equation. What makes a molecule more soluable than others ? Based only on intermolecular
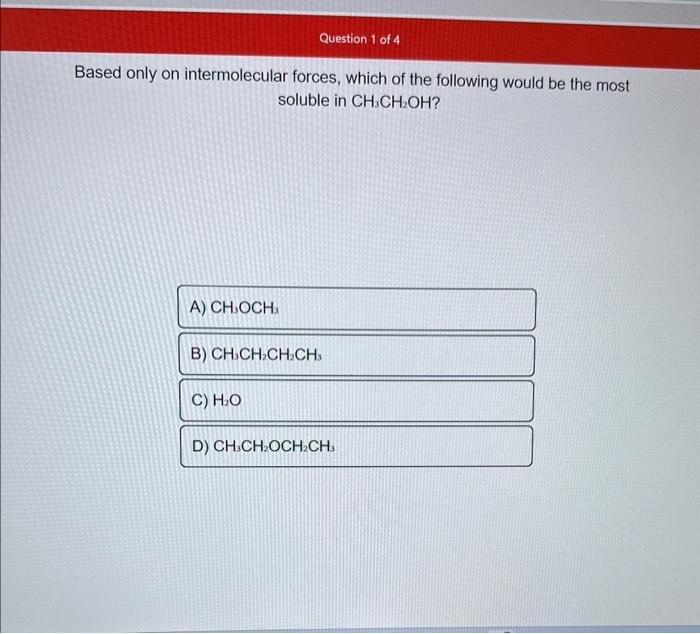
Based only on intermolecular forces, which of the following would be the most soluble in CH3CH2OH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


