Question: Can you solve this physics problem with these numbers especially the table The interatomic spring stiffness for tungsten is determined from Young's modulus measurements to
Can you solve this physics problem with these numbers especially the table
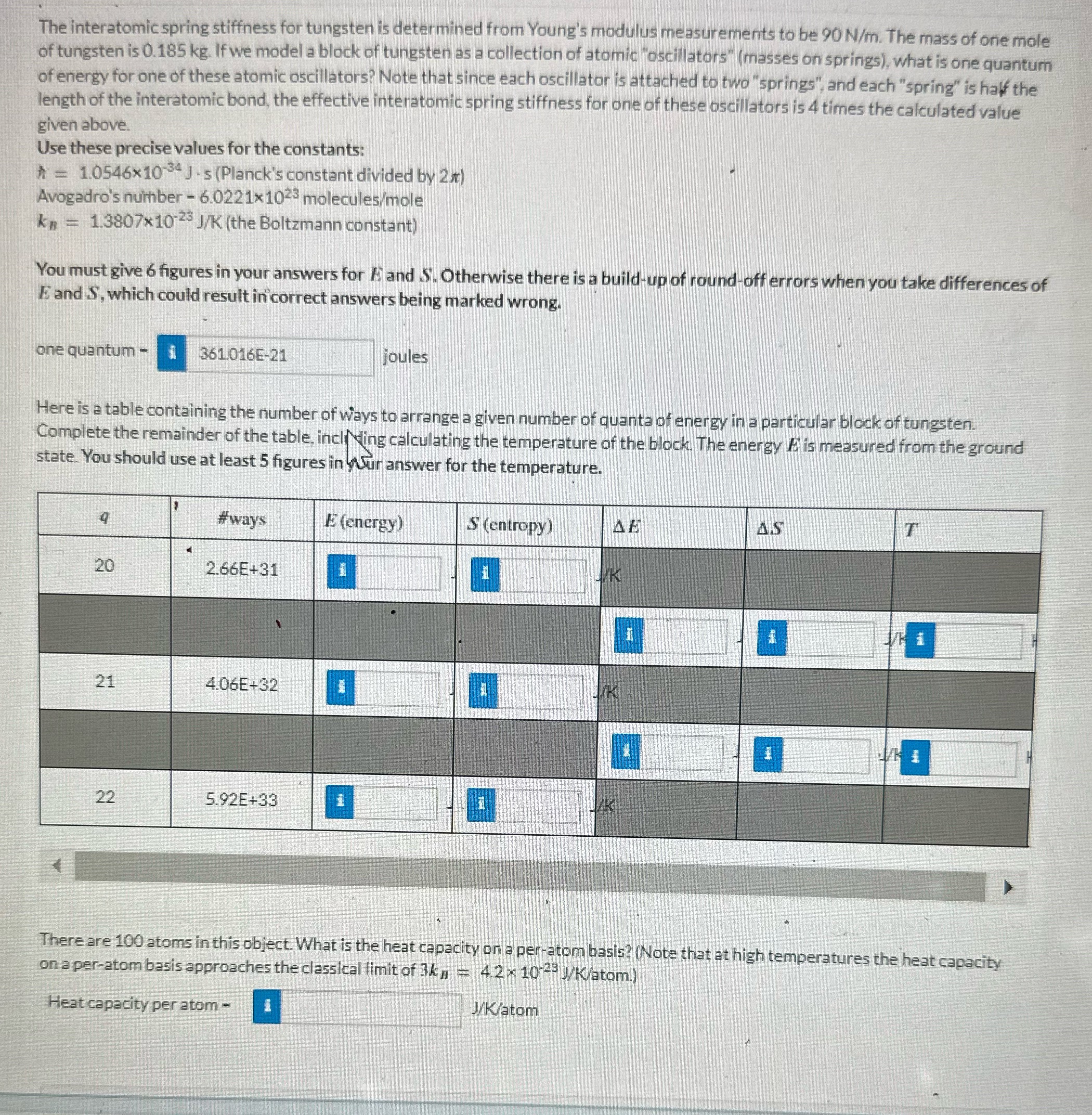
The interatomic spring stiffness for tungsten is determined from Young's modulus measurements to be 90 N/m. The mass of one mole of tungsten is 0.185 kg. If we model a block of tungsten as a collection of atomic "oscillators" (masses on springs), what is one quantum of energy for one of these atomic oscillators? Note that since each oscillator is attached to two "springs", and each "spring" is half the length of the interatomic bond, the effective interatomic spring stiffness for one of these oscillators is 4 times the calculated value given above. Use these precise values for the constants: * = 1.0546x10 * J -s (Planck's constant divided by 2x) Avogadro's number - 6.0221x1023 molecules/mole kn = 1.3807x10-23 J/K (the Boltzmann constant) You must give 6 figures in your answers for A and S'. Otherwise there is a build-up of round-off errors when you take differences of E and S, which could result in correct answers being marked wrong. one quantum - i 361 016E-21 joules Here is a table containing the number of ways to arrange a given number of quanta of energy in a particular block of tungsten. Complete the remainder of the table, including calculating the temperature of the block. The energy A is measured from the ground state. You should use at least 5 figures in your answer for the temperature. #ways E (energy) S (entropy) AE AS T 20 2.66E+31 1 1 i 21 4.06E+32 1 K 1 1 22 5.92E+33 i There are 100 atoms in this object. What is the heat capacity on a per-atom basis? (Note that at high temperatures the heat capacity on a per-atom basis approaches the classical limit of 3k, = 4.2 x 10 28 J/K/atom.) Heat capacity per atom - J/K/atom
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


