Question: CAN YOU SOLVE THIS QUESTION BY USING EXCEL TABLE? 8.3 In a chemical engineering process, water vapor (H2O) is heated to sufficiently high temperatures that
CAN YOU SOLVE THIS QUESTION BY USING EXCEL TABLE?
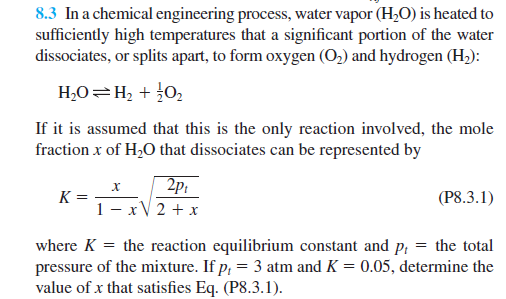
8.3 In a chemical engineering process, water vapor (H2O) is heated to sufficiently high temperatures that a significant portion of the water dissociates, or splits apart, to form oxygen (O2) and hydrogen (H2) : H2OH2+21O2 If it is assumed that this is the only reaction involved, the mole fraction x of H2O that dissociates can be represented by K=1xx2+x2pt where K= the reaction equilibrium constant and pt= the total pressure of the mixture. If pt=3atm and K=0.05, determine the value of x that satisfies Eq. (P8.3.1)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


