Question: Please solve it using python and provide the code 6 . 1 4 In a chemical engineering process, water vapor ( H 2 O )
Please solve it using python and provide the code In a chemical engineering process, water vapor
is heated to sufficiently high temperatures that a significant
portion of the water dissociates, or splits apart, to form oxy
gen and hydrogen :
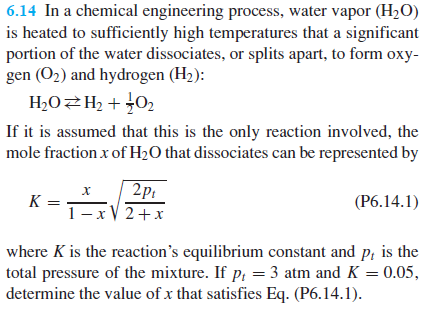
If it is assumed that this is the only reaction involved, the
mole fraction of that dissociates can be represented by
where is the reaction's equilibrium constant and is the
total pressure of the mixture. If atm and
determine the value of that satisfies EqP
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


