Question: can you write out the formulas with the calculations please! im stuck on this one 15 Question (1 point) The reaction of NO2 with ozone
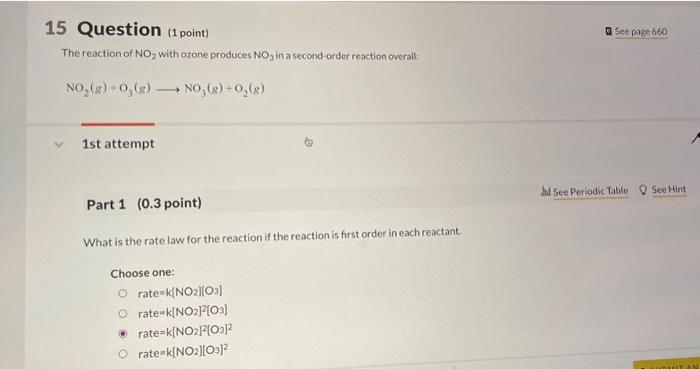
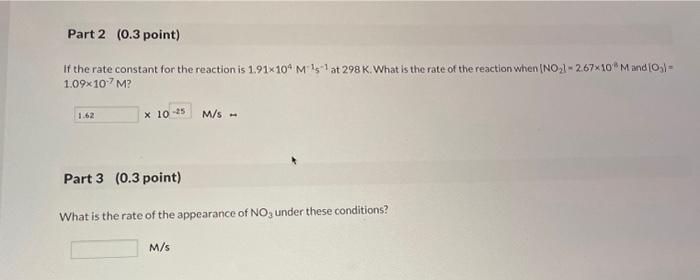
15 Question (1 point) The reaction of NO2 with ozone produces NO3 in asecond-order reaction overall: NO2(g)+O3(g)NO3(g)+O2(g) 1st attempt Part 1 (0.3 point) What is the rate law for the reaction if the reaction is first order in each reactant. Choose one: rate=k[NO2][O3]rate=k[NO2]2[O3]rate=k[NO2]2[O3]2rate=k[NO2][O3]2 If the rate constant for the reaction is 1.91104M151 at 298K. What is the rate of the reaction when [NO2]=2.67103M and [O3]= 1.09107M ? 1025M/s= Part 3 ( 0.3 point) What is the rate of the appearance of NO3 under these conditions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


