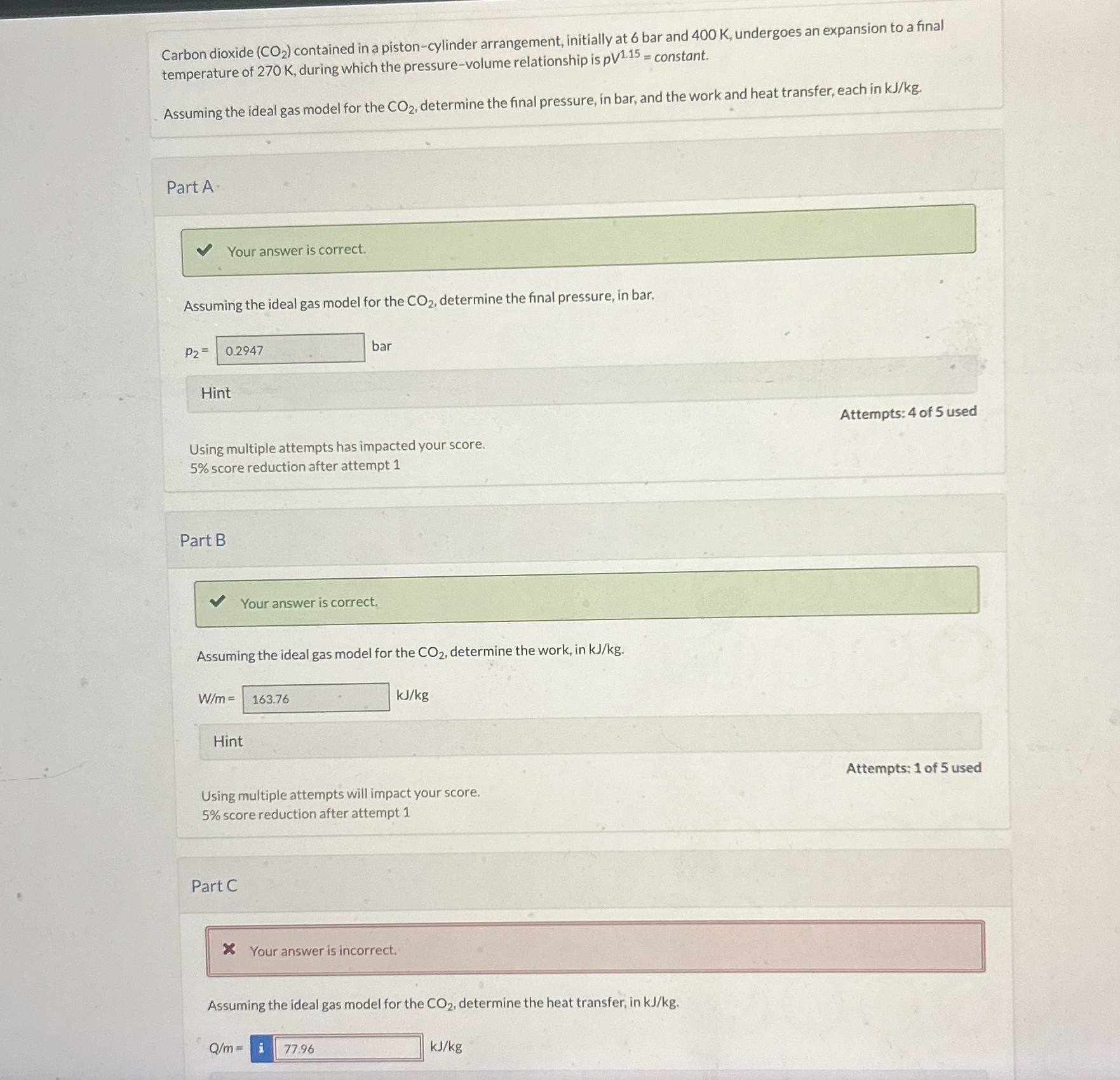
Question: Carbon dioxide ( C O 2 ) contained in a piston - cylinder arrangement, initially at 6 bar and 4 0 0 K , undergoes
Carbon dioxide contained in a pistoncylinder arrangement, initially at bar and K undergoes an expansion to a final
temperature of K during which the pressurevolume relationship is constant.
Assuming the ideal gas model for the determine the final pressure, in bar, and the work and heat transfer, each in
Part A
Your answer is correct.
Assuming the ideal gas model for the determine the final pressure, in bar.
bar
Hint
Using multiple attempts has impacted your score.
score reduction after attempt
Part B
Your answer is correct.
Assuming the ideal gas model for the determine the work, in
Hint
Using multiple attempts will impact your score.
score reduction after attempt
Part C
Your answer is incorrect.
Assuming the ideal gas model for the determine the heat transfer, in
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


