Question
Carbon monoxide is burned at 200 C under atmospheric pressure with dry air at 500 C in excess of 90% of that theoretically necessary. The
Carbon monoxide is burned at 200 °C under atmospheric pressure with dry air at 500 °C in excess of 90% of that theoretically necessary. The combustion products leave the reaction chamber at 1000 °C. Calculate the heat released in the reaction chamber in kilocalories per kg mol of CO burned, assuming complete combustion.
DATA:
Chemical reaction:
CO (g) + ½ O2 (g) = CO2 (g)
Energy balance equation:
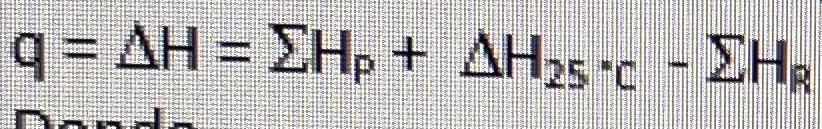
where,
P = products = CO2, O2, N2
R = reactants = CO, Air
Calculation base 1.0 kg mol
Normal heat of reaction = ΔH25°C=-67636 kcal
Heat capacity of substances
| SUBSTANCE | AVERAGE MOLAR HEAT CAPACITY between 25 -1000°C |
| CO | 7.017 |
| Air | 7.225 |
| CO2 | 11.92 |
| O2 | 7.941 |
| N2 | 7.507 |
q = = + 25c -
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access with AI-Powered Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



