Question: Carbonic acid, H2CO3, has two acidic hydrogens. A solution containing an unknown concentration of carbonic acid is titrated with potassium hydroxide. It requires 29.7mL of
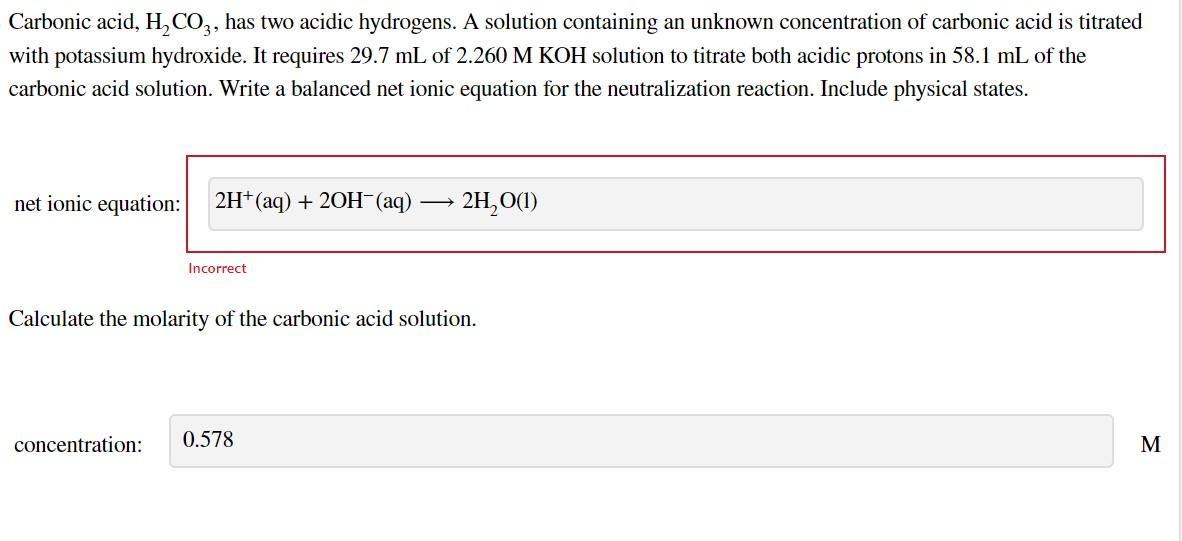
Carbonic acid, H2CO3, has two acidic hydrogens. A solution containing an unknown concentration of carbonic acid is titrated with potassium hydroxide. It requires 29.7mL of 2.260MKOH solution to titrate both acidic protons in 58.1mL of the carbonic acid solution. Write a balanced net ionic equation for the neutralization reaction. Include physical states. net ionic equation: Incorrect Calculate the molarity of the carbonic acid solution. concentration
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


