Question: Case A: Case B: ch = A + BT+CT? + D R T2 T in Kelvin The constants A, B, C, D depend on the
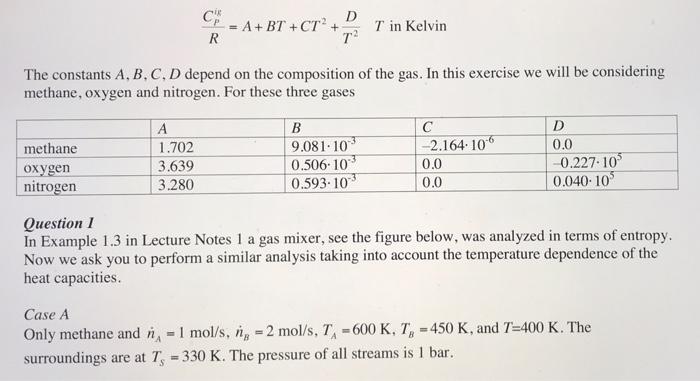


ch = A + BT+CT? + D R T2 T in Kelvin The constants A, B, C, D depend on the composition of the gas. In this exercise we will be considering methane, oxygen and nitrogen. For these three gases methane oxygen nitrogen 1.702 3.639 3.280 B 9.081.10 0.506-10 0.593.10 -2.164. 106 0.0 0.0 D 0.0 0.227.10 0,040-10 Question 1 In Example 1.3 in Lecture Notes 1 a gas mixer, see the figure below, was analyzed in terms of entropy. Now we ask you to perform a similar analysis taking into account the temperature dependence of the heat capacities. Case A Only methane and n - 1 mol/s, n, - 2 mol/s, T, =600 K, T, - 450 K, and T=400 K. The surroundings are at T-330 K. The pressure of all streams is 1 bar. Stream A is methane with n = 1 mol/s, T, = 600 K. Stream B is oxygen with ng = 1 mol/s, T, = 750 K. and T=500 K. The surroundings are at T-330 K. The pressure of all streams is 1 bar. For both Case A and Case B: determine the rate at which entropy is being produced (symbol Sa
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


