Question: Chapter 9 : Kinetics and mechanisms : solvent exchange 9 . 1 What mechanisms do the following activation parameters for solvent exchange suggest? The rate
Chapter : Kinetics and mechanisms : solvent exchange
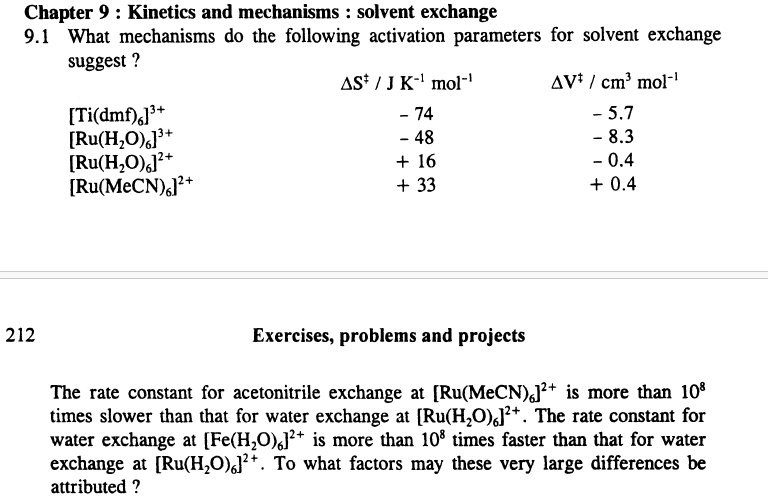
What mechanisms do the following activation parameters for solvent exchange
suggest?
The rate constant for acetonitrile exchange at is more than
times slower than that for water exchange at The rate constant for
water exchange at is more than times faster than that for water
exchange at To what factors may these very large differences be
attributed?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


