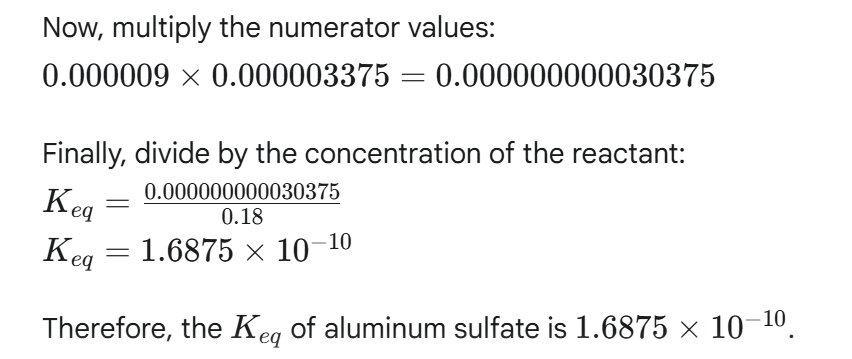
Question: check final answer for q17 Now, multiply the numerator values: 0.000009 x 0.000003375 = 0.000000000030375 Finally, divide by the concentration of the reactant: __ Q.000000000030375
check final answer for q17
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


