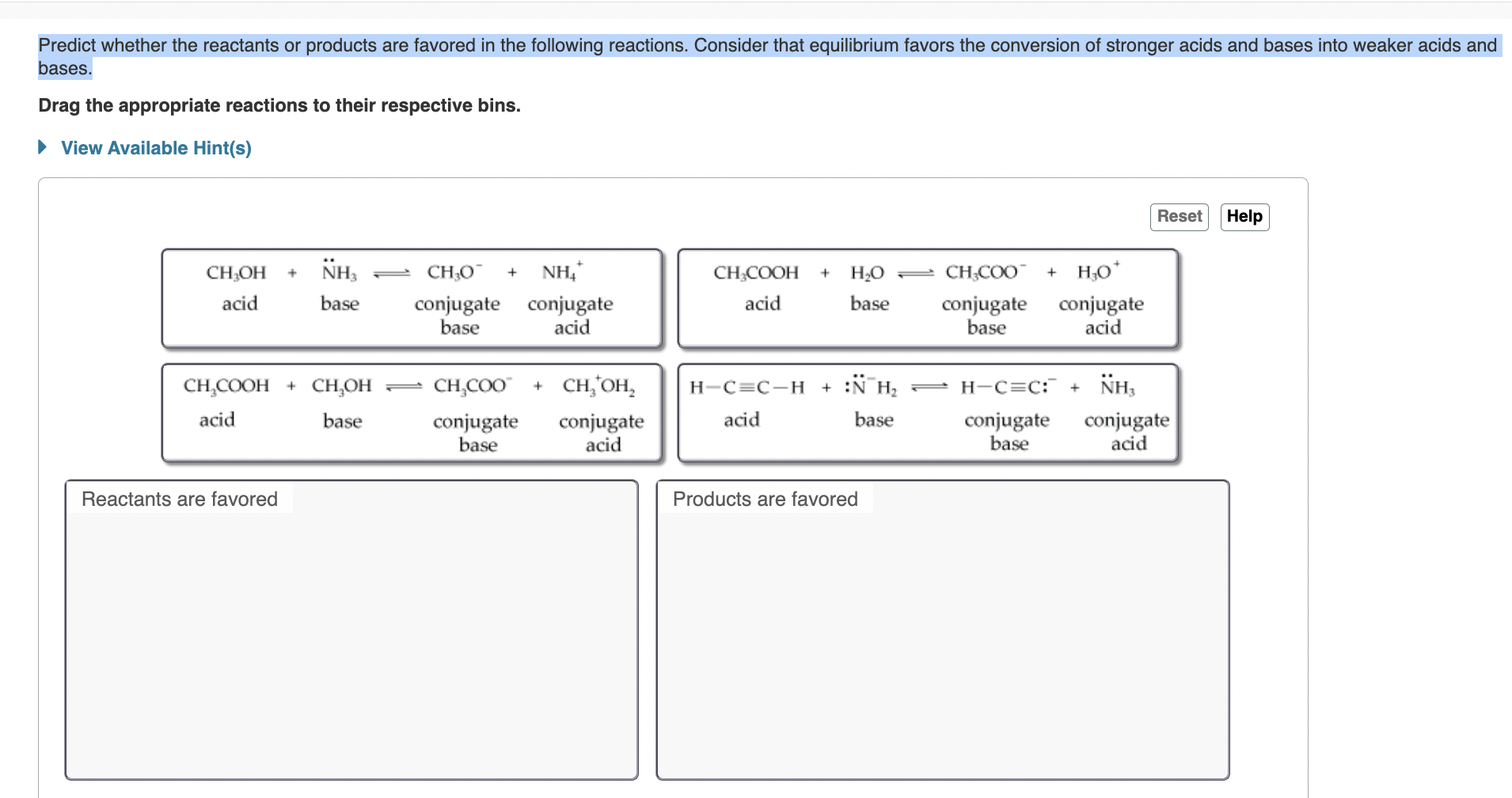
Question: check image Predict whether the reactants or products are favored in the following reactions. Consider that equilibrium favors the conversion of stronger acids and bases
 check image
check image
Predict whether the reactants or products are favored in the following reactions. Consider that equilibrium favors the conversion of stronger acids and bases into weaker acids and bases. Drag the appropriate reactions to their respective bins. View Available Hint(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


