Question: Chemical Engineering finding the net and gross calorific value Question One A pulverized coal sample has the following analysis by weight. %C %H2 %CH4 %N2
Chemical Engineering finding the net and gross calorific value
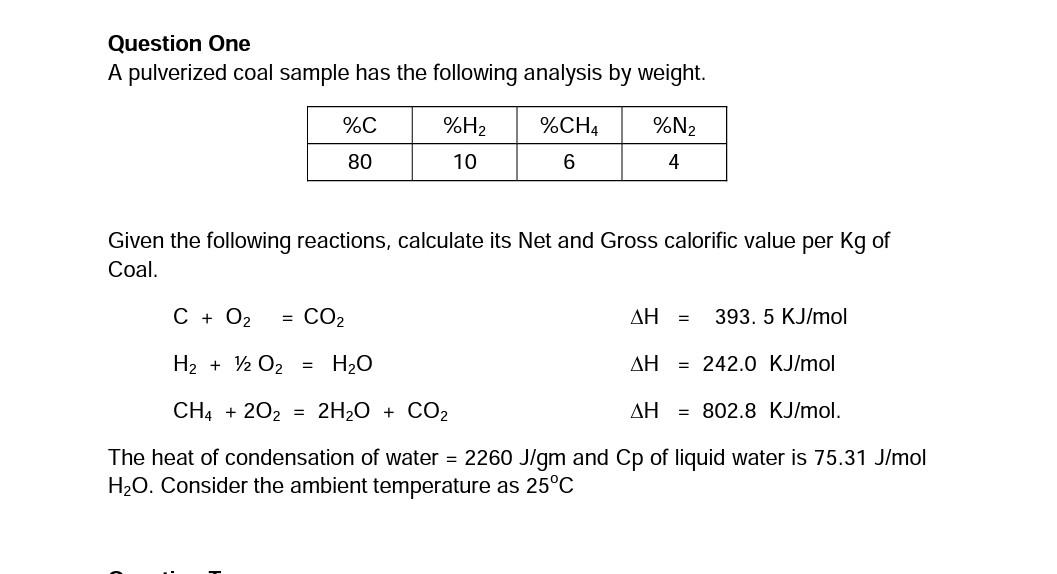
Question One A pulverized coal sample has the following analysis by weight. %C %H2 %CH4 %N2 80 10 6 4 Given the following reactions, calculate its Net and Gross calorific value per kg of Coal. C + O2 = CO2 AH = 393. 5 KJ/mol H2 + 12 O2 = H2O AH = 242.0 KJ/mol CH4 + 202 = 2H2O + CO2 AH = 802.8 KJ/mol. The heat of condensation of water = 2260 J/gm and Cp of liquid water is 75.31 J/mol H20. Consider the ambient temperature as 25C
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


