Question: Chemical equations emulate algebraic equations. Common terms on both sides of an equation can be cancelled. This method is frequently used to convert total ionic
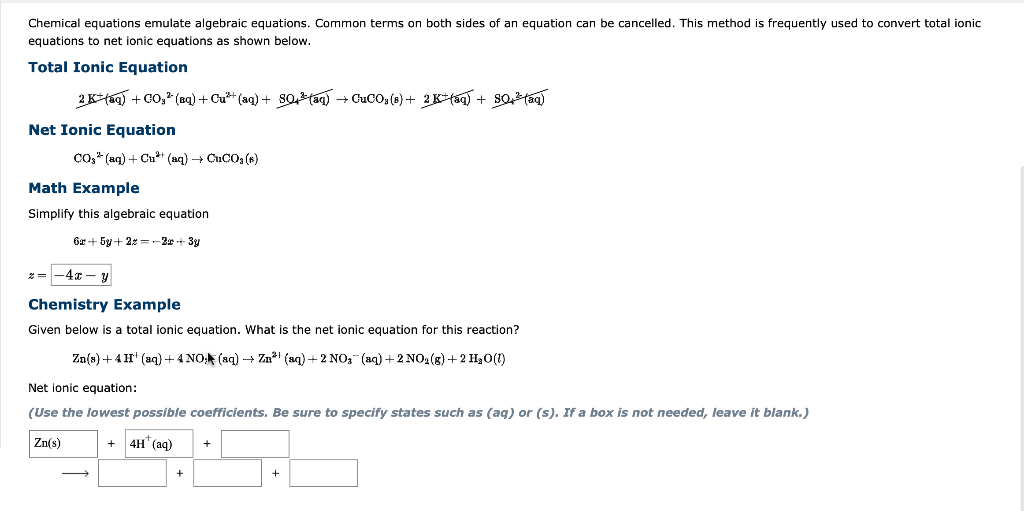
Chemical equations emulate algebraic equations. Common terms on both sides of an equation can be cancelled. This method is frequently used to convert total ionic equations to net ionic equations as shown below. Total Ionic Equation 2K+(aq)+CO92(qq)+Cu2+(aq)+SO+(aq)CuCO3(s)+2K+(aq)+SO42(aq) Net Ionic Equation CO32(aq)+Cu2+(aq)CuCO3(k) Math Example Simplify this algebraic equation 6x+5y+2z=2x+3y z= Chemistry Example Given below is a total ionic equation. What is the net ionic equation for this reaction? Zn(s)+4H+(aq)+4NO/(aq)Zn2+(aq)+2NO3(aq)+2NO2(g)+2HH2O(l) Net ionic equation: (Use the lowest possible coefficients, Be sure to specify states such as (aq) or (s), If a box is not needed, leave it blank.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


