Question: Chemical Reaction Engineering Question 5.For the reaction A->R, second-order kinetics and Cao = 4 mollliter, we get 50% conversion after 2.5 hour in a batch
Chemical Reaction Engineering Question
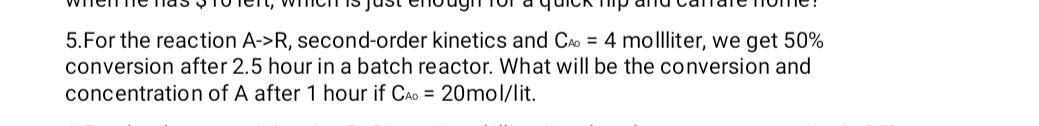
5.For the reaction A->R, second-order kinetics and Cao = 4 mollliter, we get 50% conversion after 2.5 hour in a batch reactor. What will be the conversion and concentration of A after 1 hour if Cao = 20mol/lit
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


