Question: Chemical Reaction Engineering question. Please help me solve these questions with complete handwritting solutions. Please try not to take answers from other tutors. Thank you.
Chemical Reaction Engineering question. Please help me solve these questions with complete handwritting solutions. Please try not to take answers from other tutors. Thank you. Have a nice day :)
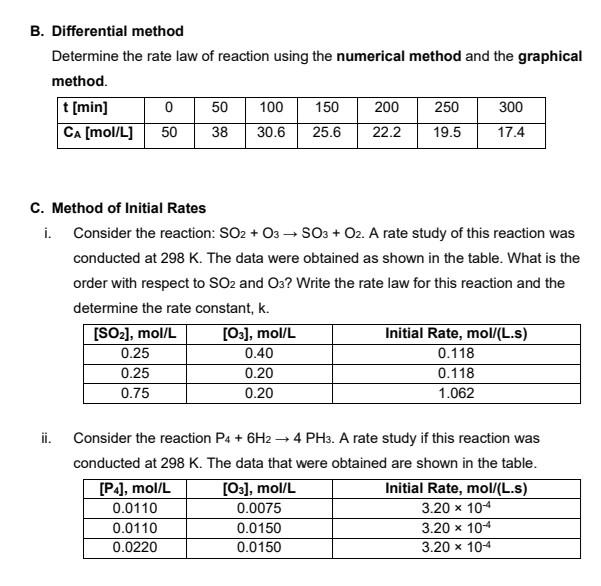
B. Differential method Determine the rate law of reaction using the numerical method and the graphical method. t[min] 50 100 150 200 250 300 CA [mol/L] 38 30.6 25.6 22.2 19.5 17.4 1930 C. Method of Initial Rates i. Consider the reaction: SO2 + 03 SO3 + O2. A rate study of this reaction was conducted at 298 K. The data were obtained as shown in the table. What is the order with respect to SO2 and O3? Write the rate law for this reaction and the determine the rate constant, k. [SO2), mol/L [O3), mol/L Initial Rate, mol/(L.s) 0.25 0.40 0.118 0.25 0.20 0.118 0.75 0.20 1.062 ii. - Consider the reaction P4 + 6H2 + 4 PH3. A rate study if this reaction was conducted at 298 K. The data that were obtained are shown in the table. [P], mol/L [O3], mol/L Initial Rate, mol/(L.s) 0.0110 0.0075 3.20 x 10-4 0.0110 0.0150 3.20 x 10+ 0.0220 0.0150 3.20 x 10-4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


