Question: CHEMICAL REACTION STOICHIOMETRY - Multiple Reactions, yield, and Selectivity: Example: Consider the reactions C2H6C2H6+H2C2H4+H22CH4 or Excel Online Select PDF File take place in a continuous
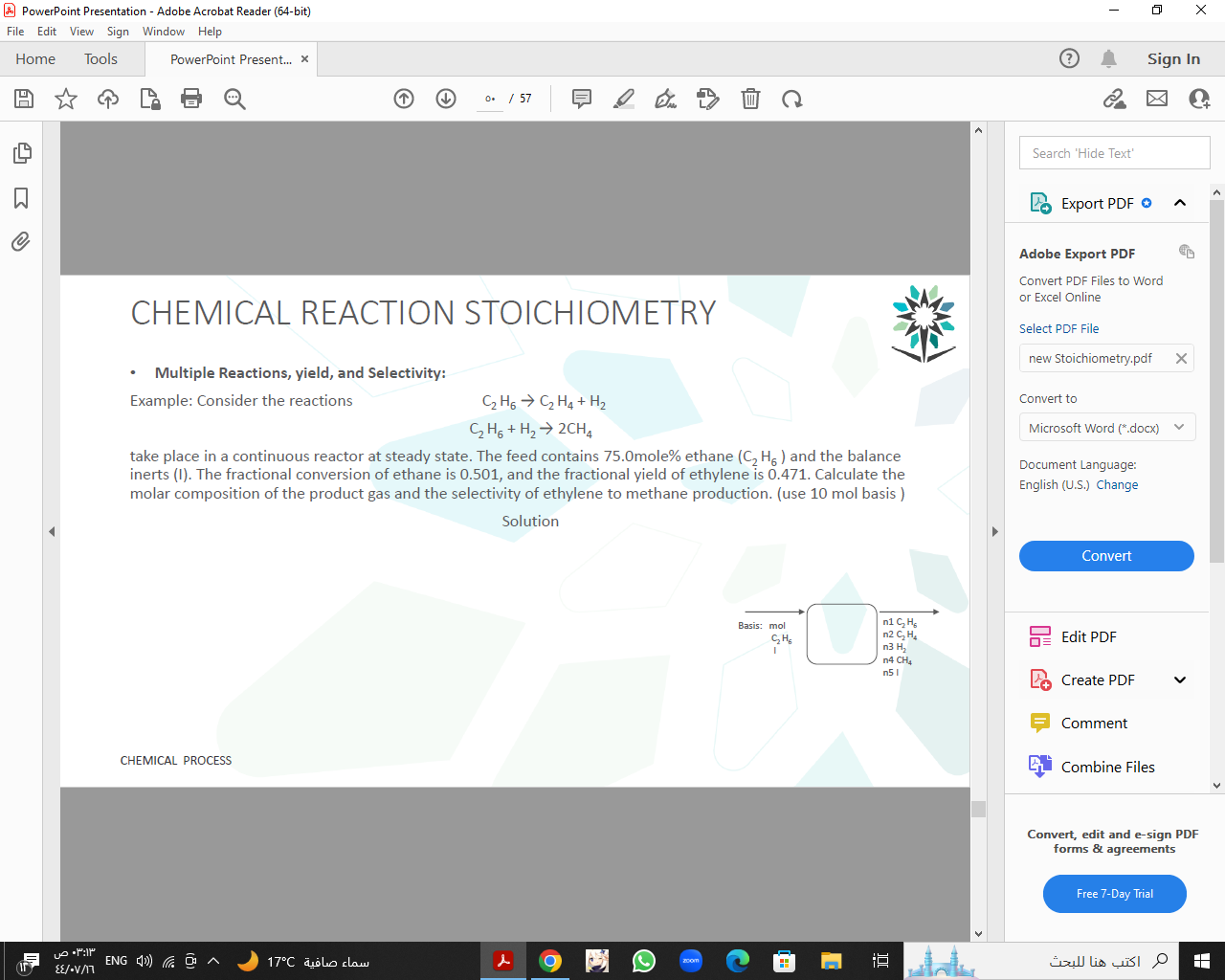
CHEMICAL REACTION STOICHIOMETRY - Multiple Reactions, yield, and Selectivity: Example: Consider the reactions C2H6C2H6+H2C2H4+H22CH4 or Excel Online Select PDF File take place in a continuous reactor at steady state. The feed contains 75.0 mole\% ethane (C2H6) and the balance inerts (I). The fractional conversion of ethane is 0.501, and the fractional yield of ethylene is 0.471. Calculate the molar composition of the product gas and the selectivity of ethylene to methane production. (use 10 mol basis ) Document Language: English (U.S.) Change Edit PDF Create PDF Comment Combine Files Convert, edit and e-sign PDF forms & agreements
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


