Question: 2. (15 pts) Coal-fired power plants and some metallurgical plants that treat sulfide ores or concentrates generate SO2, which if emitted to the atmosphere
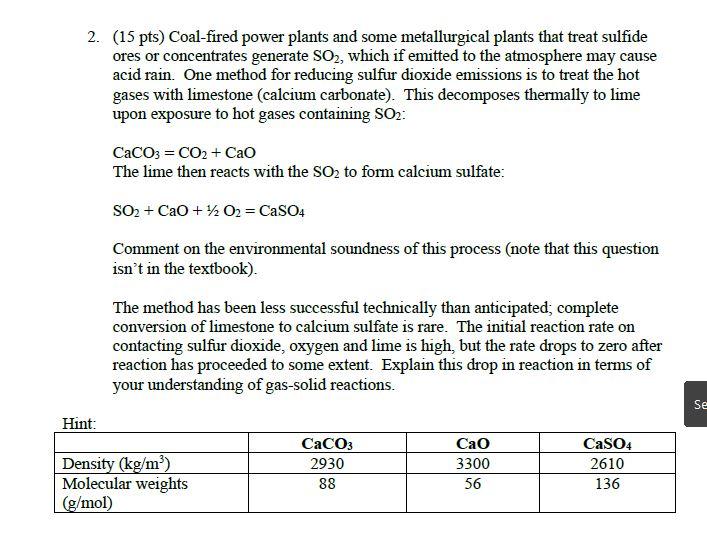
2. (15 pts) Coal-fired power plants and some metallurgical plants that treat sulfide ores or concentrates generate SO2, which if emitted to the atmosphere may cause acid rain. One method for reducing sulfur dioxide emissions is to treat the hot gases with limestone (calcium carbonate). This decomposes thermally to lime upon exposure to hot gases containing SO: Hint: CaCO3 = CO2 + CaO The lime then reacts with the SO to form calcium sulfate: SO + CaO + 1/2O2 = CaSO4 Comment on the environmental soundness of this process (note that this question isn't in the textbook). The method has been less successful technically than anticipated; complete conversion of limestone to calcium sulfate is rare. The initial reaction rate on contacting sulfur dioxide, oxygen and lime is high, but the rate drops to zero after reaction has proceeded to some extent. Explain this drop in reaction in terms of your understanding of gas-solid reactions. Density (kg/m) Molecular weights (g/mol) CaCO3 2930 88 CaO 3300 56 CaSO4 2610 136 Se
Step by Step Solution
3.34 Rating (163 Votes )
There are 3 Steps involved in it
2 CCO CO The lime then reacts with the so to from calcium suffate 50 CO 10 C 504 process The a... View full answer

Get step-by-step solutions from verified subject matter experts


