Question: The condensation reaction of acetone in aqueous solution occurs when a base B reacts reversibly with acetone to form a carbanion C3H5O. The carbanion
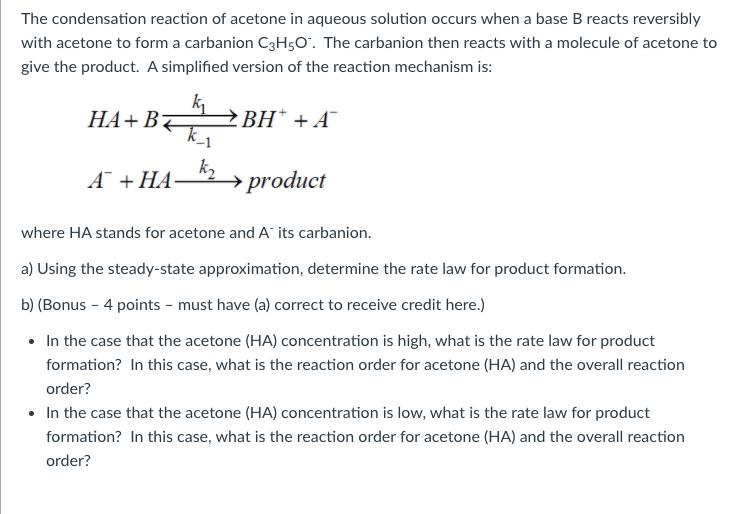
The condensation reaction of acetone in aqueous solution occurs when a base B reacts reversibly with acetone to form a carbanion C3H5O. The carbanion then reacts with a molecule of acetone to give the product. A simplified version of the reaction mechanism is: + k + A k, + product where HA stands for acetone and A its carbanion. a) Using the steady-state approximation, determine the rate law for product formation. b) (Bonus - 4 points - must have (a) correct to receive credit here.) In the case that the acetone (HA) concentration is high, what is the rate law for product formation? In this case, what is the reaction order for acetone (HA) and the overall reaction order? In the case that the acetone (HA) concentration is low, what is the rate law for product formation? In this case, what is the reaction order for acetone (HA) and the overall reaction order?
Step by Step Solution
3.42 Rating (168 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


