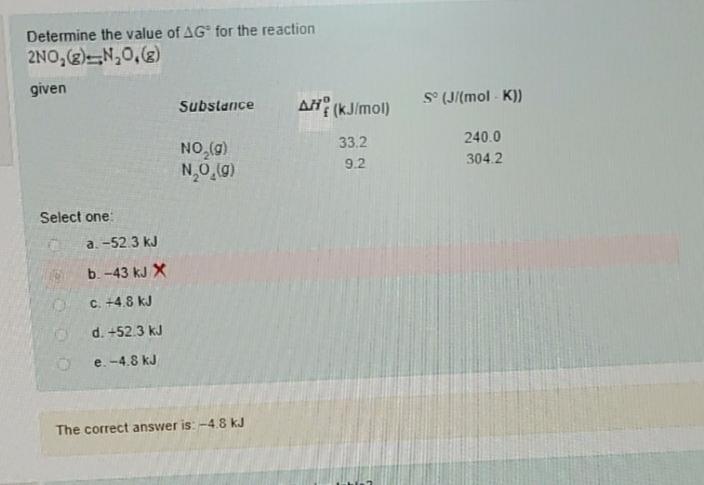
Question: Chemistry Determine the value of AG for the reaction 2NO(g) N0,(g) given Substance NO(g) NO(g) Select one: a.-52.3 kJ b. -43 kJ X C.+4.8 K
Chemistry
Determine the value of AG for the reaction 2NO(g) N0,(g) given Substance NO(g) NO(g) Select one: a.-52.3 kJ b. -43 kJ X C.+4.8 K d. +52.3 kJ e. -4.8 kJ The correct answer is: -4.8 kJ AH(kJ/mol) 33.2 9.2 S (J/(mol-K)) 240.0 304.2
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
Step 1 Gibbs free energy is calculated by using enthalpy and entropy as follows Step 2 But her... View full answer

Get step-by-step solutions from verified subject matter experts


