Question: Chemistry equilibrium question. Why am i getting this wrong? I've tried both and it still says it's wrong even when I follow the format. Construct
Chemistry equilibrium question.
Why am i getting this wrong? I've tried both and it still says it's wrong even when I follow the format.
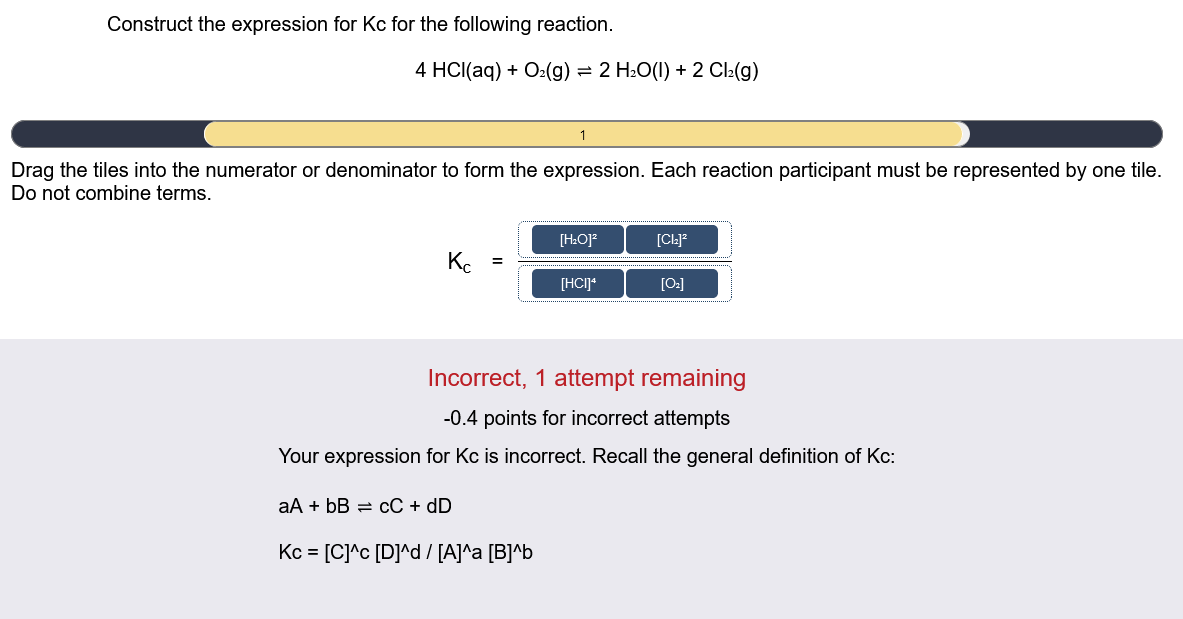
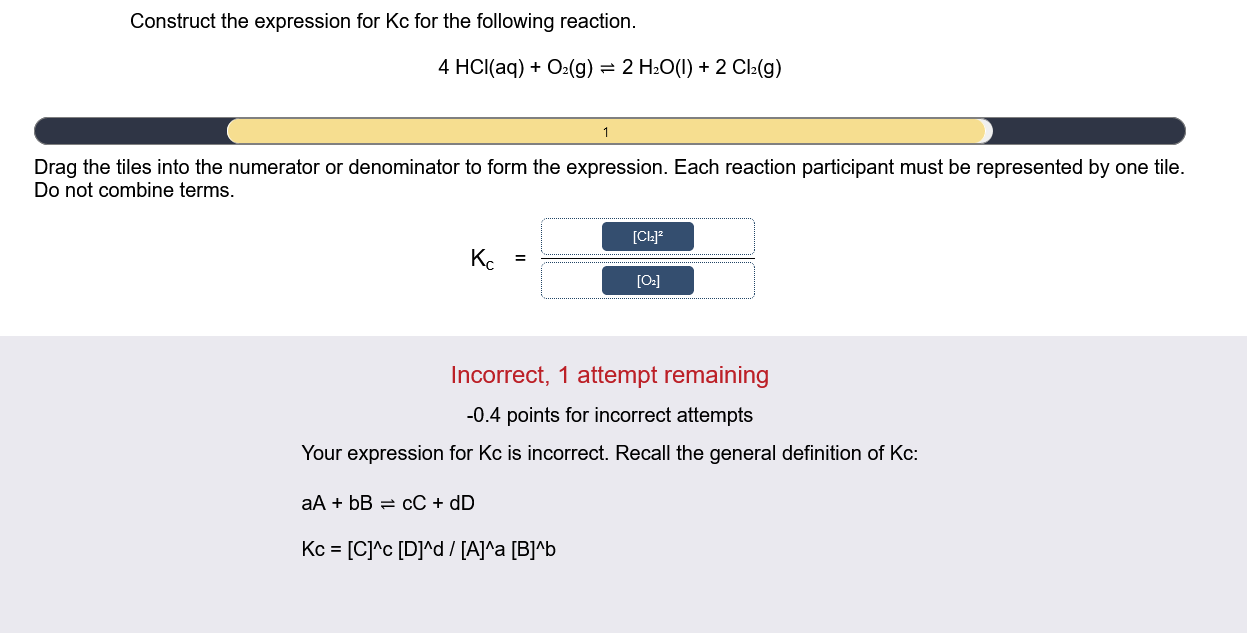
Construct the expression for Kc for the following reaction. 4HCl(aq)+O2(g)2H2O(l)+2Cl2(g) Drag the tiles into the numerator or denominator to form the expression. Each reaction participant must be represented by one tile. Do not combine terms. Kc= Incorrect, 1 attempt remaining 0.4 points for incorrect attempts Your expression for Kc is incorrect. Recall the general definition of Kc : aA+bBcC+dDKc=[C]c[D]d/[A]a[B]b Construct the expression for Kc for the following reaction. 4HCl(aq)+O2(g)2H2O(l)+2Cl2(g) Drag the tiles into the numerator or denominator to form the expression. Each reaction participant must be represented by one tile. Do not combine terms. Incorrect, 1 attempt remaining 0.4 points for incorrect attempts Your expression for Kc is incorrect. Recall the general definition of Kc : aA+bBcC+dDKc=[C]c[D]d/[A]a[B]b
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


