Question: What is the limiting reactant? How can you tell based on your observations? Using the table provided in appendix H, look up the solubility

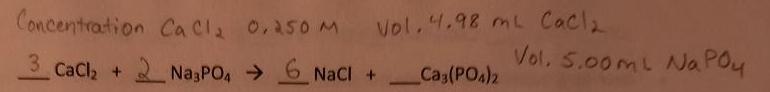
What is the limiting reactant? How can you tell based on your observations? Using the table provided in appendix H, look up the solubility values for the two products NaCl: Ca3(PO4)2 Based on the solubility numbers, what would the solid product be? Concentration CaCla 0,250 vol. 4.98 mL Cacl2 Vol. 5.00mL Na Poy 3 CaCl, + 2Na3PO, 6 NaCI + Cas(PO)2
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
What is the Limiting Reactant The limiting reagent is the reactant that is used up completely This stops the reaction and no further products are made ... View full answer

Get step-by-step solutions from verified subject matter experts


