Question: Calculate the formal potential, E, for the given reaction. NO, (aq) + 3 H* (aq) + 2 e= HNO, (aq) + H, O(1) E
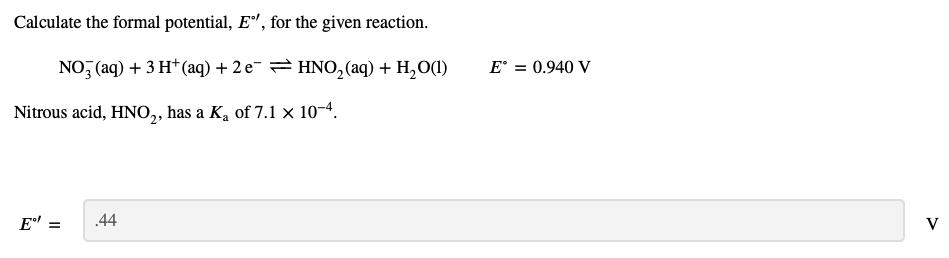
Calculate the formal potential, E", for the given reaction. NO, (aq) + 3 H* (aq) + 2 e= HNO, (aq) + H, O(1) E = 0.940 V Nitrous acid, HNO,, has a K, of 7.1 x 10-4. E = .44 V
Step by Step Solution
★★★★★
3.43 Rating (153 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (2 attachments)
636476cb5e17e_238514.pdf
180 KBs PDF File
636476cb5e17e_238514.docx
120 KBs Word File


