Balance each of the following unbalanced equations; then calculate the standard potential, E, and decide whether each
Question:
Balance each of the following unbalanced equations; then calculate the standard potential, E°, and decide whether each is product-favored at equilibrium as written. (All reactions are carried out in acid solution.)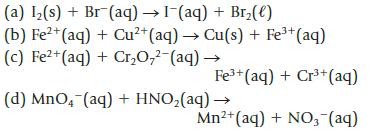
Transcribed Image Text:
(a) I₂(s) + Br (aq) → 1-(aq) + Br₂(e) (b) Fe²+ (aq) + Cu²+ (aq) → Cu(s) + Fe³+ (aq) (c) Fe²+ (aq) + Cr₂O₂² (aq) → Fe³+ (aq) + Cr³+ (aq) (d) MnO4 (aq) + HNO₂(aq) → Mn²+ (aq) + NO3- (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
To balance these unbalanced redox equations and determine whether they are productfavored at equilibrium you can follow the steps of balancing redox r...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following unbalanced equations; then calculate the standard potential, E, and decide whether each is product-favored at equilibrium as written. (All reactions are carried out in...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Considering these data where 'P1' estimates are analyst forecasts of future stock prices: Stock PO P1 A B C D B 52.5 59 0.4 1.1 24.5 29 0.26 2.1 36.4 43 0.23 1.5 38 42 0.33 1.4 Market Risk Premium...
-
The following data relate to labor cost for production of 12,500 cellular telephones: Actual: 13,600 hours at $16.15 .... $219,640 Standard: 13,725 hours at $16.00 ... $219,600 a. Determine the rate...
-
Assume that Acorn Inc. sold bonds with a face value of $100,000 for $104,000. Was the market interest rate equal to, less than, or greater than the bonds contractual interest rate? Explain.
-
What does the unit GSE represent on a demodulated spectrum? Is it relevant to other vibration parameters?
-
Why is CVP analysis generally used as a short-run tool? Would CVP ever be appropriate as a long-run model?
-
3. Starting a Brazilian Ju Jitsu (BJJ) studio Lisa has practiced BJJ over the past 2 years and although she is only a blue belt, she thinks she knows enough to turn her hobby into a business. While...
-
Consider the following half-reactions: (a) Based on E values, which metal is the most easily oxidized? (b) Which metals on this list are capable of reducing Fe 2+ (aq) to Fe(s)? (c) Write a balanced...
-
Calculate the value of E for each of the following reactions. Decide whether each is product-favored at equilibrium in the direction written. [Reaction (d) is carried out in basic solution.] (a)...
-
Southwire Company and Essex Group, Inc. are direct competitors in the cable and wire industry. Southwire's logistics system is a warehouse organizational system with components extending from...
-
Read the section in Chapter 12: Challenges to Our Understanding of Leadership that begins on page 418. Research and explore some of the new theories of leadership using some of the articles below (or...
-
consider what you would do at your company as the newly hired HR manager-- One department manager is worried about an employee's outbursts and high level of anger while at work Another department...
-
Discuss the need for both parents in understanding pregnancy and being involved. What is your view on childbirth classes and birth plans? Please include an example for your reasons. Research infant...
-
Using the following words to write a legible paragraph which ensure me that the student understands Human Resources Training and Development. Use all the words to in the paragraph. Readiness for...
-
Some describe China's culture as "success-obsessed" which is incompatible with the prevalence of failure in startup ventures. Most other types of financing models, only a few experienced investors...
-
An economic theory suggests that as people become richer they tend to have more children. Analyze the relationship between income (INCOME) and number of children (CHILDS) to test the theory.
-
Write a paper about medication error system 2016.
-
A block of mass M 1 = 3.0 kg rests on top of a second block of mass M 2 - 5.0 kg, and the second block sits on a surface that is so slippery that the friction can be assumed to be zero (see Fig....
-
Two spheres, one of balsa wood (diameter 40 cm) and one of steel (diameter 10 cm), have the same mass. (a) How many times greater is the terminal speed of the steel sphere than that of the wooden...
-
A car is outfitted with a flat piece of plywood mounted vertically on its front bumper. As seen in Figure P3.91, a block of wood is simply placed in front of the car just as the car begins to...
-
In the development of the appropriate audit plan, it is important that the internal auditor obtain sufficient and appropriate audit evidence to understand the implications of fraud that might impact...
-
On January 1, 2024, Syth, Inc. purchased several industrial forklifts for use in its warehouse. The forklifts had a list price of $205,000. The seller agreed to allow a 3.00 percent discount because...
-
Question 2 (38 Marks) Mike is employed by Cookie Inc., a CCPC in the technology industry as a sales manager. He has requested your assistance in preparing his 2022 income tax return and provided you...

Study smarter with the SolutionInn App


