Question: CHEN 231 - Class Activity Answer the following: 1. For the following methanation reaction, calculate the viscosity of a feed gas mixture made of the
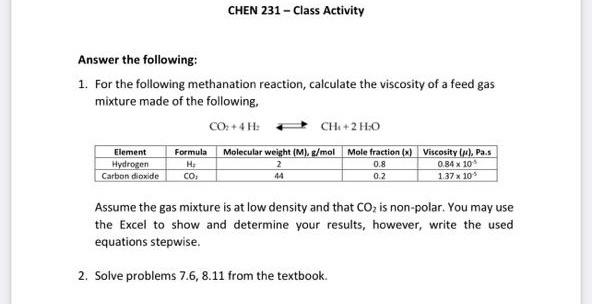
CHEN 231 - Class Activity Answer the following: 1. For the following methanation reaction, calculate the viscosity of a feed gas mixture made of the following. CO+ 4H CHE+2 Formula Molecular weight (M), g/mol Mole fraction (x) Viscosity (p), Pais Hydrogen Hy 0.84% 10 Carbon dioxide CO. 1.3710 Element 0.8 44 02 Assume the gas mixture is at low density and that CO2 is non-polar. You may use the Excel to show and determine your results, however, write the used equations stepwise. 2. Solve problems 7.6, 8.11 from the textbook
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


