Question: Choose an expression for the equilibrium constant (Kc) for the following chemical equation: 4HCl(g)+O2(g)2H2O(l)+2Cl2(g) Kc=[HCl]4[O2][Cl2]2[H2O]2Kc=[Cl2]2[H2O]2[HCl]4[O2]Kc=[HCl]4[O2][Cl2]2Kc=[Cl2]2[HCl]4[O2]
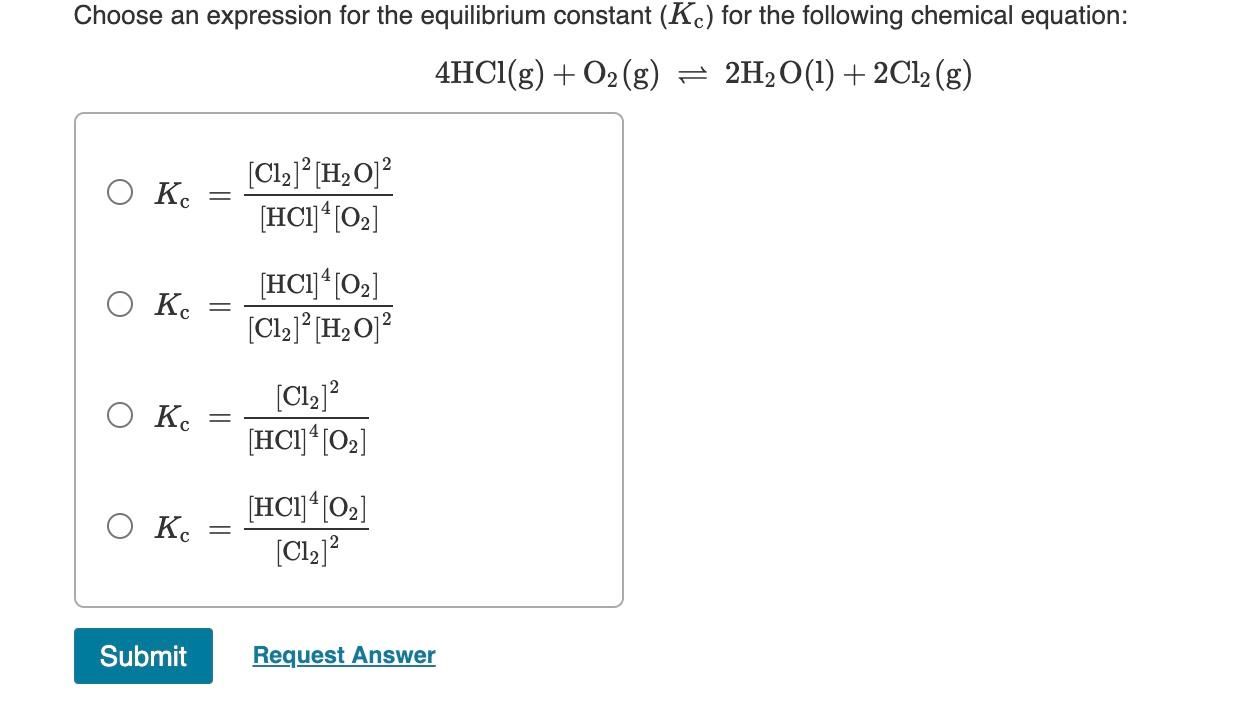
Choose an expression for the equilibrium constant (Kc) for the following chemical equation: 4HCl(g)+O2(g)2H2O(l)+2Cl2(g) Kc=[HCl]4[O2][Cl2]2[H2O]2Kc=[Cl2]2[H2O]2[HCl]4[O2]Kc=[HCl]4[O2][Cl2]2Kc=[Cl2]2[HCl]4[O2]
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


