Question: solve this example in matlab and also plot for conversion vs volume and reaction rate va volume. Example +7 Gas-Phase Reaction in a Microreactor-Molar Flow
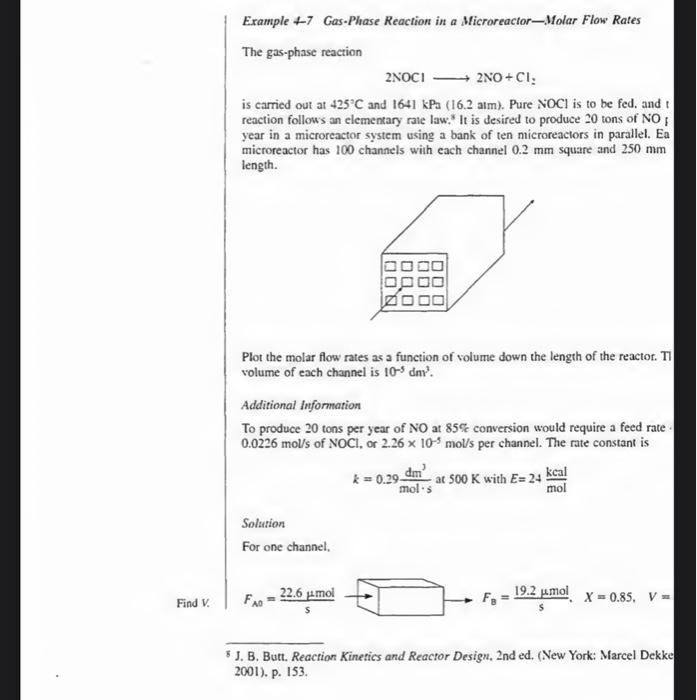
Example +7 Gas-Phase Reaction in a Microreactor-Molar Flow Rates The gas-phase reaction 2NOCl2NO+Cl2 is carried out at 425C and 1641kPa(16.2atm). Pure NOCl is to be fed. and t reaction follows an elementary rate law: It is desired to produce 20 tons of NO year in a microreactor system using a bank of ten microreaclors in parallel. Ea microreactor has 100 channels with each channel 0.2mm square and 250mm length. Plot the molar flow rates as a function of volume down the length of the reactor. T7 volume of each channel is 105dm3. Additional Information To produce 20 tons per year of NO at 85% conversion would require a feed rate 0.0226mol/s of NOCl, or 2.26105mol/s per channel. The rate constant is k=0.29mol3dm3at500KwithE=24molkcal Solution For one channel, 5. B. Butt. Reaction Kinetics and Reactor Design, 2nd ed. (New York: Marcel Dekke 2001), p. 153
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


