Question: Choose the correct electron dot structure for the molecule C2H20. The position of the atoms are constant for all answer options but the number of
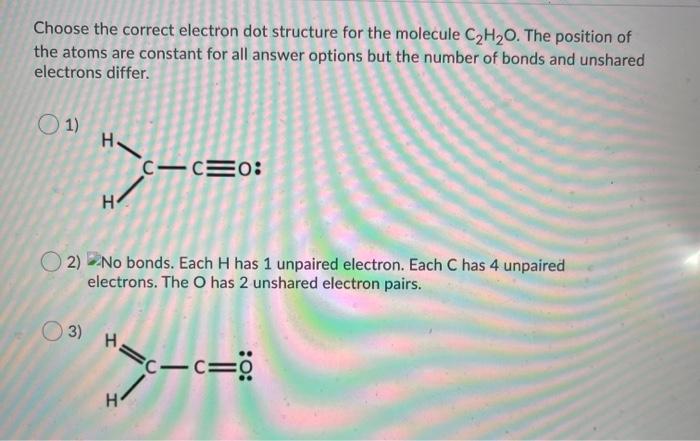
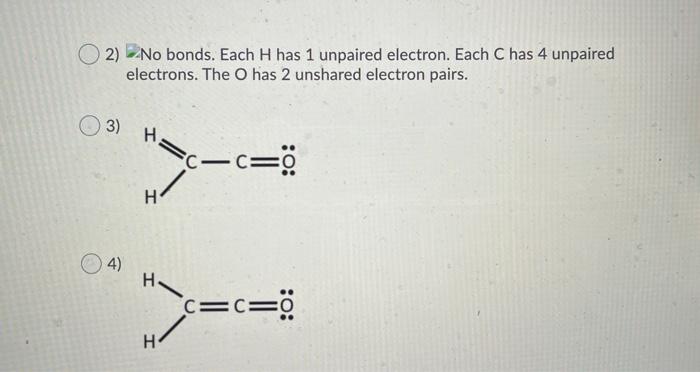
Choose the correct electron dot structure for the molecule C2H20. The position of the atoms are constant for all answer options but the number of bonds and unshared electrons differ. 1) H- C-CEO: H 2) No bonds. Each H has 1 unpaired electron. Each C has 4 unpaired electrons. The O has 2 unshared electron pairs. 03) H=c- c= :0: H 2) No bonds. Each H has 1 unpaired electron. Each C has 4 unpaired electrons. The O has 2 unshared electron pairs. 3) H C-CEO c H 4) C=C=0 =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


