Question: Choose the one correct or one incorrect statement regarding chromatography: gravity can be used as a delivery system the mobile phase can be a


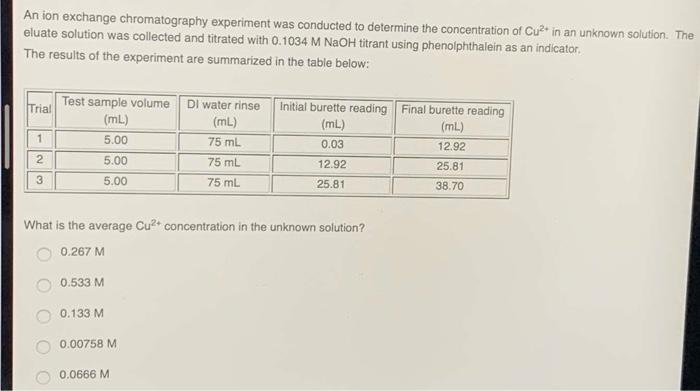

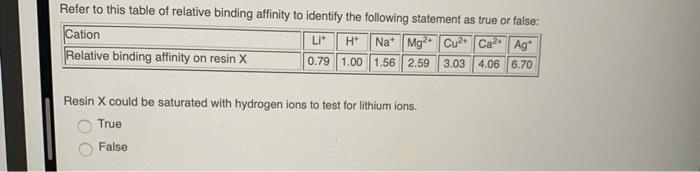

Choose the one correct or one incorrect statement regarding chromatography: gravity can be used as a delivery system the mobile phase can be a gas the chromatographic bed can be a resin the chromatographic bed can be a glass sheet the stationary phase can be a liquid Choose the one correct or one incorrect statement regarding the technique of ion exchange chromatography: the resin is a soluble salt with a net charge which can bind ions of opposite charge ions in the sample mixture are separated based on their charges this technique uses the reversible interchange of ions between the mobile and stationary phases an ion with a lower binding affinity will be displaced from the resin binding sites by an ion with higher binding affinity neutral ions will not interact with the resin binding sites and will elute from the column quickly An ion exchange chromatography experiment was conducted to determine the concentration of Cu+ in an unknown solution. The eluate solution was collected and titrated with 0.1034 M NaOH titrant using phenolphthalein as an indicator. The results of the experiment are summarized in the table below: Trial 1 2 3 Test sample volume (mL) 5.00 5.00 5.00 0.133 M What is the average Cu+ concentration in the unknown solution? 0.267 M 0.533 M 0.00758 M DI water rinse (mL) 75 mL 75 mL 75 mL 0.0666 M Initial burette reading Final burette reading (mL) (mL) 0.03 12.92 12.92 25.81 25.81 38.70 7 + Refer to this table of relative binding affinity to identify the following statement as true or false: Lit H Na Mg2 Cu Ca+ Ag 0.79 1.00 1.56 2.59 3.03 4.06 6.70 Cation Relative binding affinity on resin X Resin X is a cation exchange resin. True False DE ysic Refer to this table of relative binding affinity to identify the following statement as true or false: H+ Na Mg2+ Cu2+ Ca+ Ag Lit 0.79 1.00 1.56 2.59 3.03 4.06 6.70 Cation Relative binding affinity on resin X Resin X could be saturated with hydrogen ions to test for lithium ions. True False A waste solution containing copper ions was analyzed using ion exchange chromatography and titration, and it was determined that the solution has a copper ion concentration of 0.1242 M. The waste solution needs to be treated with sodium sulphide, NaS, to precipitate the copper from solution as copper sulphide, CuS, according to the following reaction: Cu2+ + NaS-> CuS + 2Na+ What mass of NaS must be added, per litre of waste solution, to react fully with the copper present? Molar mass of Cu+: 63.546 g/mol Molar mass of NaS: 78.045 g/mol 0.1954 g 15.25 g 7.892 g 9.693 g 0.07892 g
Step by Step Solution
3.53 Rating (163 Votes )
There are 3 Steps involved in it
The detailed answer for the above question is provided below Question 1 Chromatography is a versatile separation technique used in various fields to separate and analyze complex mixtures of substances ... View full answer

Get step-by-step solutions from verified subject matter experts


