Question: Choose the transition (in a hydrogen atom) below that represents the emission of the shortest wavelength photon. n=3ton=7 n=8ton=12 n=3ton=1 n=1ton=2 n=4ton=3 Place the following
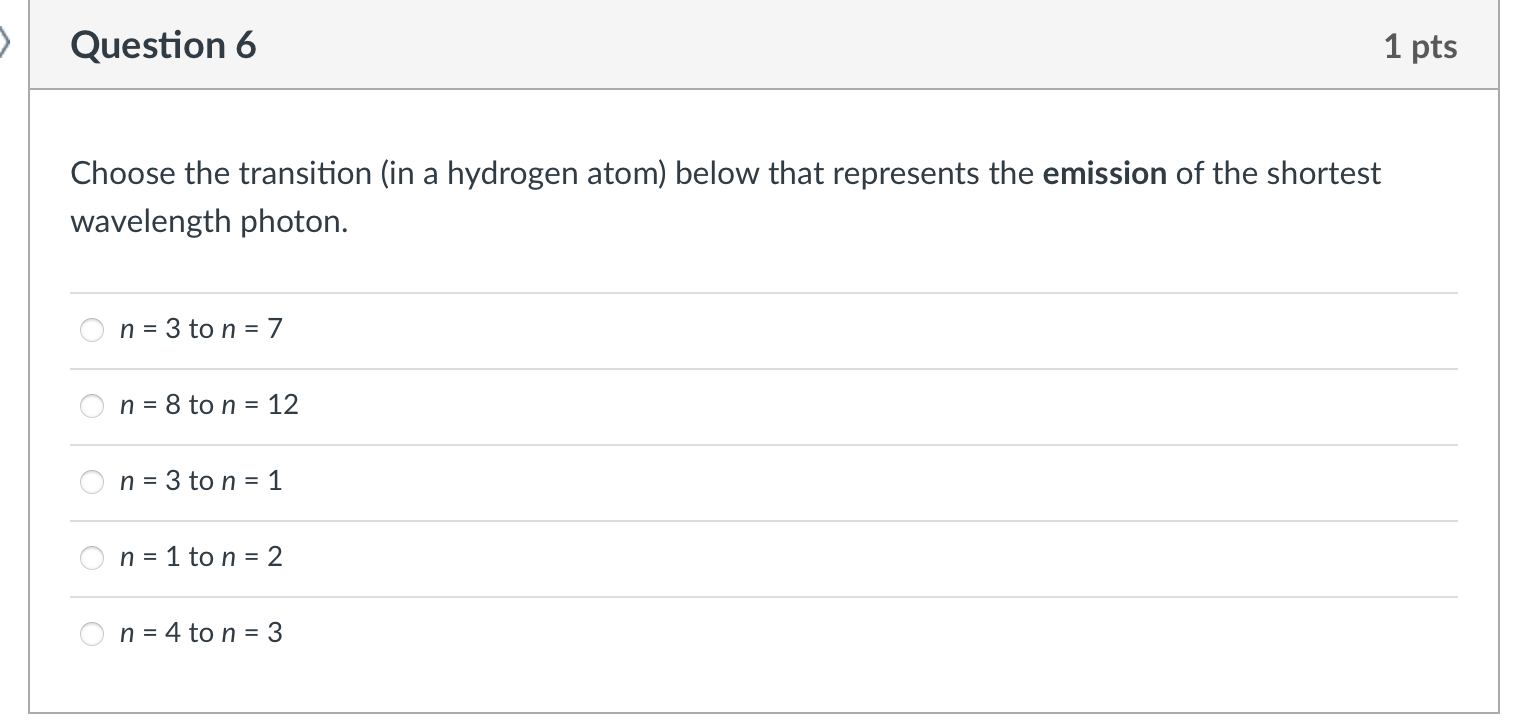
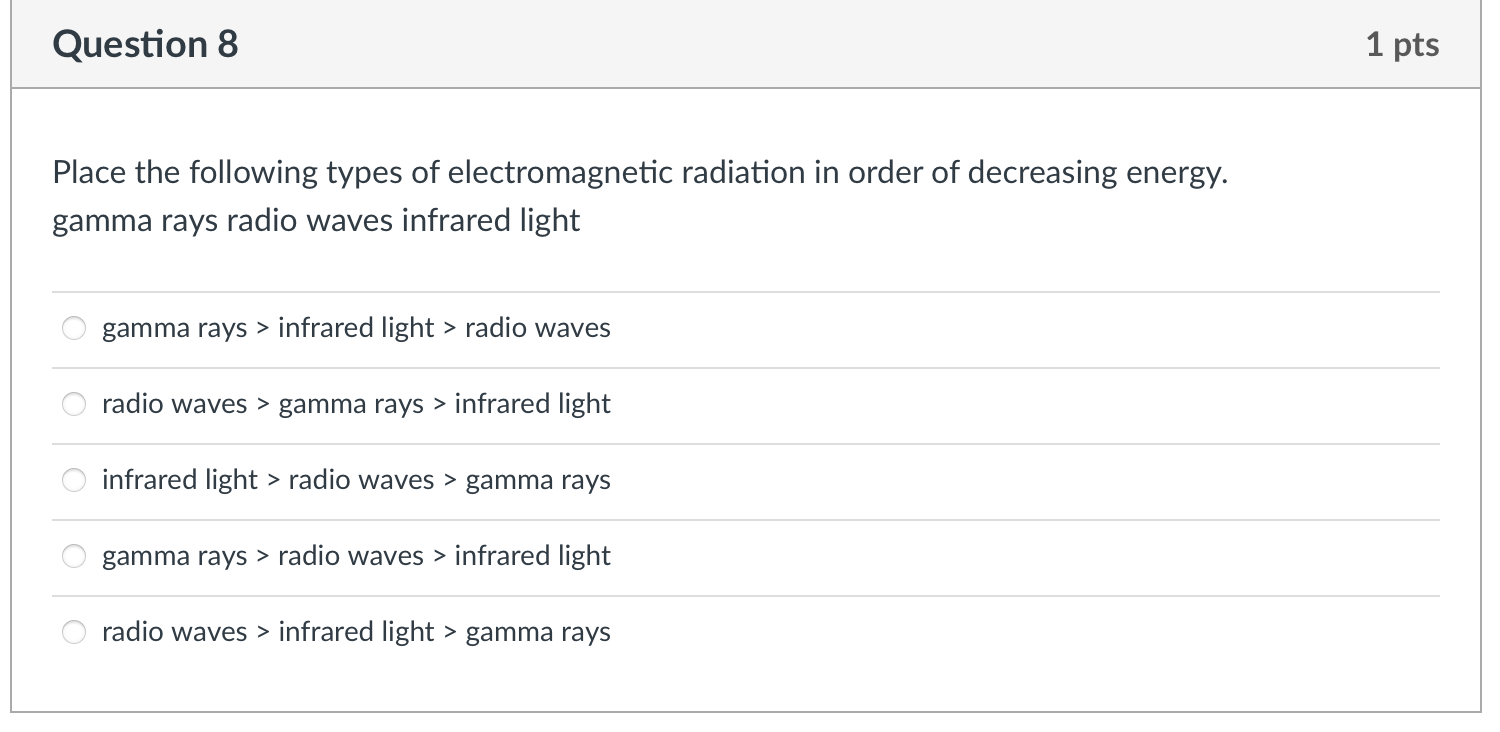
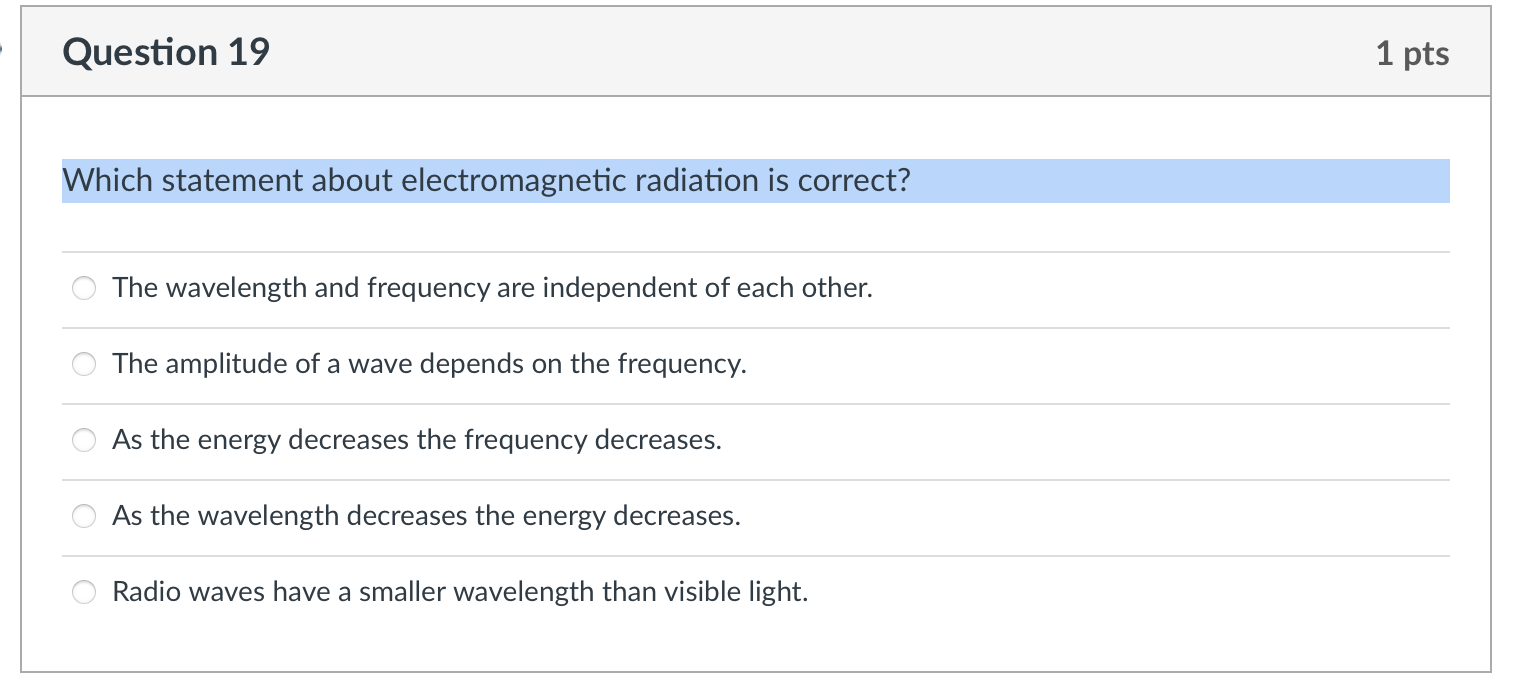
Choose the transition (in a hydrogen atom) below that represents the emission of the shortest wavelength photon. n=3ton=7 n=8ton=12 n=3ton=1 n=1ton=2 n=4ton=3 Place the following types of electromagnetic radiation in order of decreasing energy. gamma rays radio waves infrared light gamma rays > infrared light > radio waves radio waves > gamma rays > infrared light infrared light > radio waves > gamma rays gamma rays > radio waves > infrared light radio waves > infrared light > gamma rays Which statement about electromagnetic radiation is correct? The wavelength and frequency are independent of each other. The amplitude of a wave depends on the frequency. As the energy decreases the frequency decreases. As the wavelength decreases the energy decreases. Radio waves have a smaller wavelength than visible light
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


