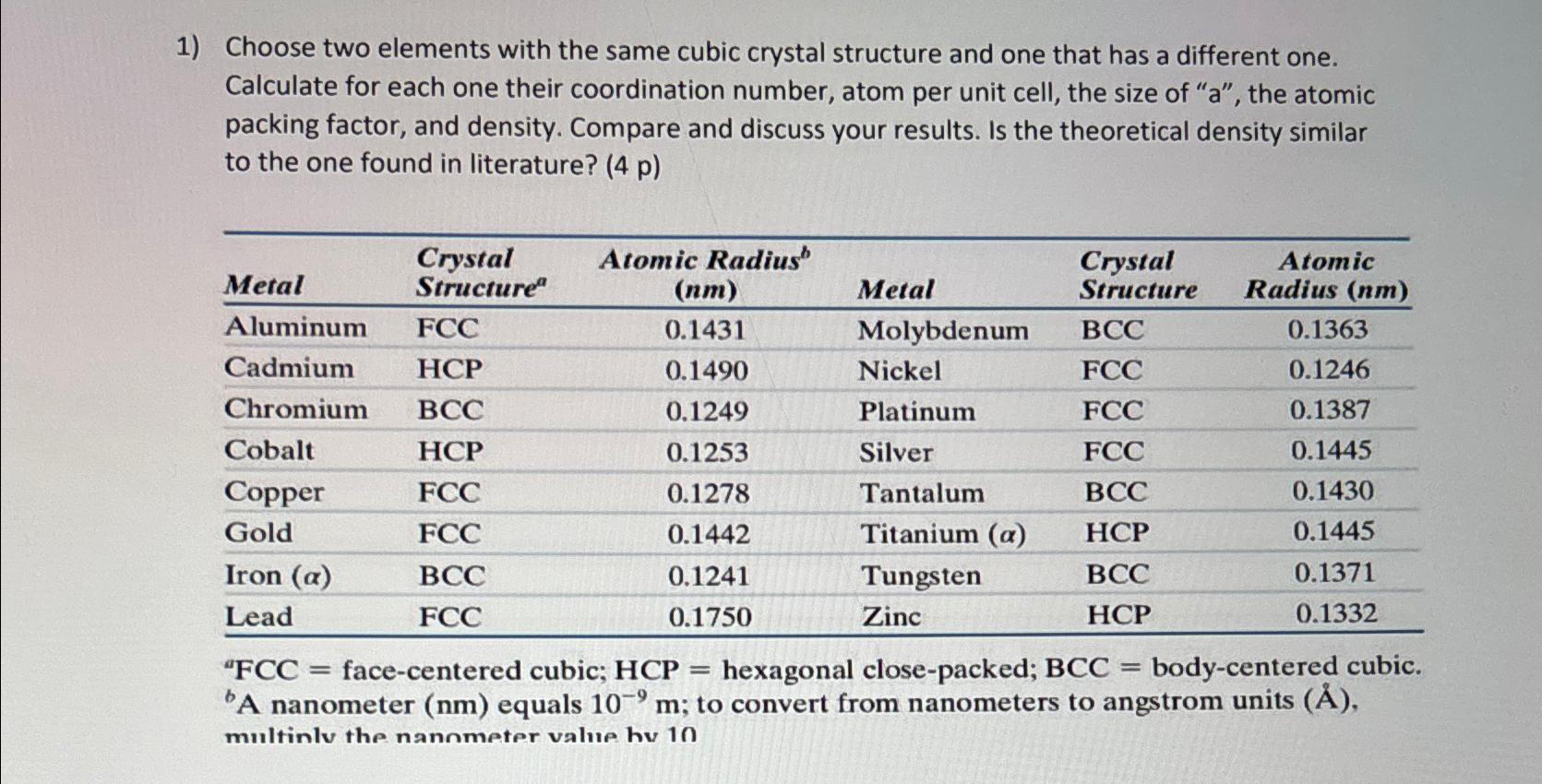
Question: Choose two elements with the same cubic crystal structure and one that has a different one. Calculate for each one their coordination number, atom per
Choose two elements with the same cubic crystal structure and one that has a different one. Calculate for each one their coordination number, atom per unit cell, the size of the atomic packing factor, and density. Compare and discuss your results. Is the theoretical density similar to the one found in literature?
tableMetaltableCrystalStructuretableAtomic Radius
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


