Question: clear solution, please Problem 4: Chlorine (Cl)is prepared by oxidation of hydrogen chloride (HCl)gas in the presence of a catalyst at 723K to produce water
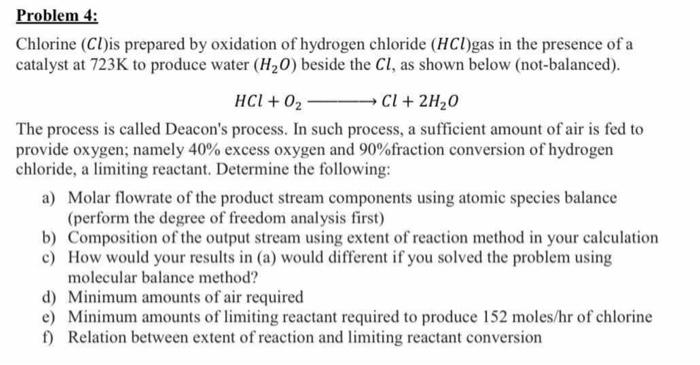
Problem 4: Chlorine (Cl)is prepared by oxidation of hydrogen chloride (HCl)gas in the presence of a catalyst at 723K to produce water (H20) beside the Cl, as shown below (not-balanced). HCI + O2 Cl + 2H20 The process is called Deacon's process. In such process, a sufficient amount of air is fed to provide oxygen; namely 40% excess oxygen and 90%fraction conversion of hydrogen chloride, a limiting reactant. Determine the following: a) Molar flowrate of the product stream components using atomic species balance (perform the degree of freedom analysis first) b) Composition of the output stream using extent of reaction method in your calculation c) How would your results in (a) would different if you solved the problem using molecular balance method? d) Minimum amounts of air required e) Minimum amounts of limiting reactant required to produce 152 moles/hr of chlorine f) Relation between extent of reaction and limiting reactant conversion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


