Question: clear solution, please Problem 3: The reaction between ethylene (C2H,) and hydrogen bromide (HBr) forms bromoethane (C2H5Br) at constant temperature and in presence of a
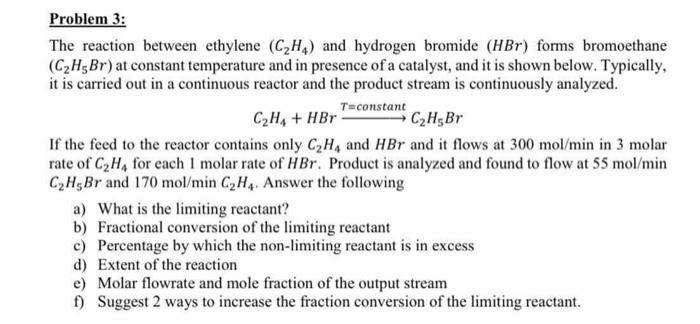
Problem 3: The reaction between ethylene (C2H,) and hydrogen bromide (HBr) forms bromoethane (C2H5Br) at constant temperature and in presence of a catalyst, and it is shown below. Typically, it is carried out in a continuous reactor and the product stream is continuously analyzed. T=constant C2H4 + HBr C2H5Br If the feed to the reactor contains only C2H4 and HBr and it flows at 300 mol/min in 3 molar rate of C, H, for each 1 molar rate of HBr. Product is analyzed and found to flow at 55 mol/min CH5Br and 170 mol/min C, H. Answer the following a) What is the limiting reactant? b) Fractional conversion of the limiting reactant c) Percentage by which the non-limiting reactant is in excess d) Extent of the reaction e) Molar flowrate and mole fraction of the output stream 1) Suggest 2 ways to increase the fraction conversion of the limiting reactant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


