Question: In table below, there are descriptions of an experiment on samples of three different chemical compounds. Decide whether the compound is ionic or molecular,
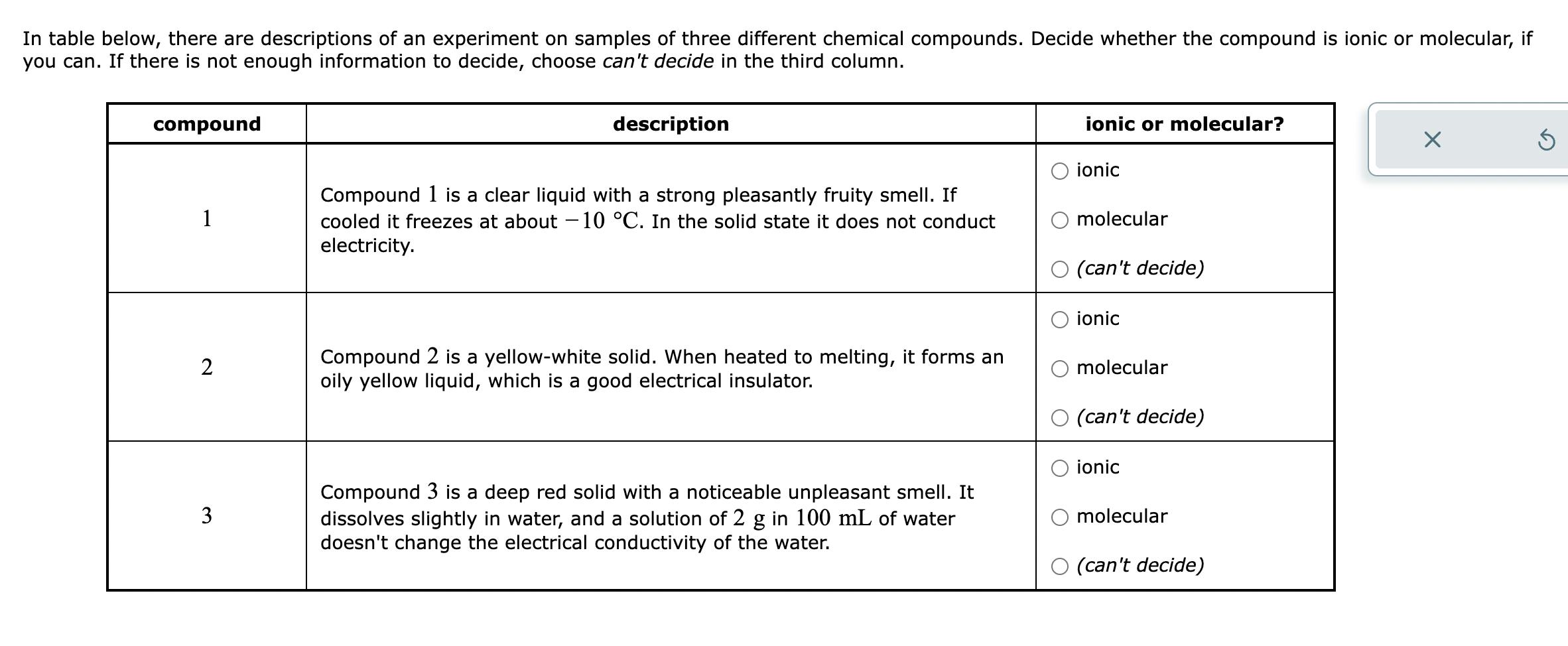
In table below, there are descriptions of an experiment on samples of three different chemical compounds. Decide whether the compound is ionic or molecular, if you can. If there is not enough information to decide, choose can't decide in the third column. compound 1 2 3 description Compound 1 is a clear liquid with a strong pleasantly fruity smell. If cooled it freezes at about -10 C. In the solid state it does not conduct electricity. Compound 2 is a yellow-white solid. When heated to melting, it forms an oily yellow liquid, which is a good electrical insulator. Compound 3 is a deep red solid with a noticeable unpleasant smell. It dissolves slightly in water, and a solution of 2 g in 100 mL of water doesn't change the electrical conductivity of the water. ionic or molecular? ionic molecular O (can't decide) ionic molecular (can't decide) ionic molecular O (can't decide) X
Step by Step Solution
3.55 Rating (162 Votes )
There are 3 Steps involved in it
Youre correct that Compound 1 is most likely molecular and Compound 3 is most likely ionic However t... View full answer

Get step-by-step solutions from verified subject matter experts


