Question: Colligative Properties (ALEKS lab) Introduction Lab Data Verify your mass calculation Verify your molality calculation Did you account for the freezing-point of your DI
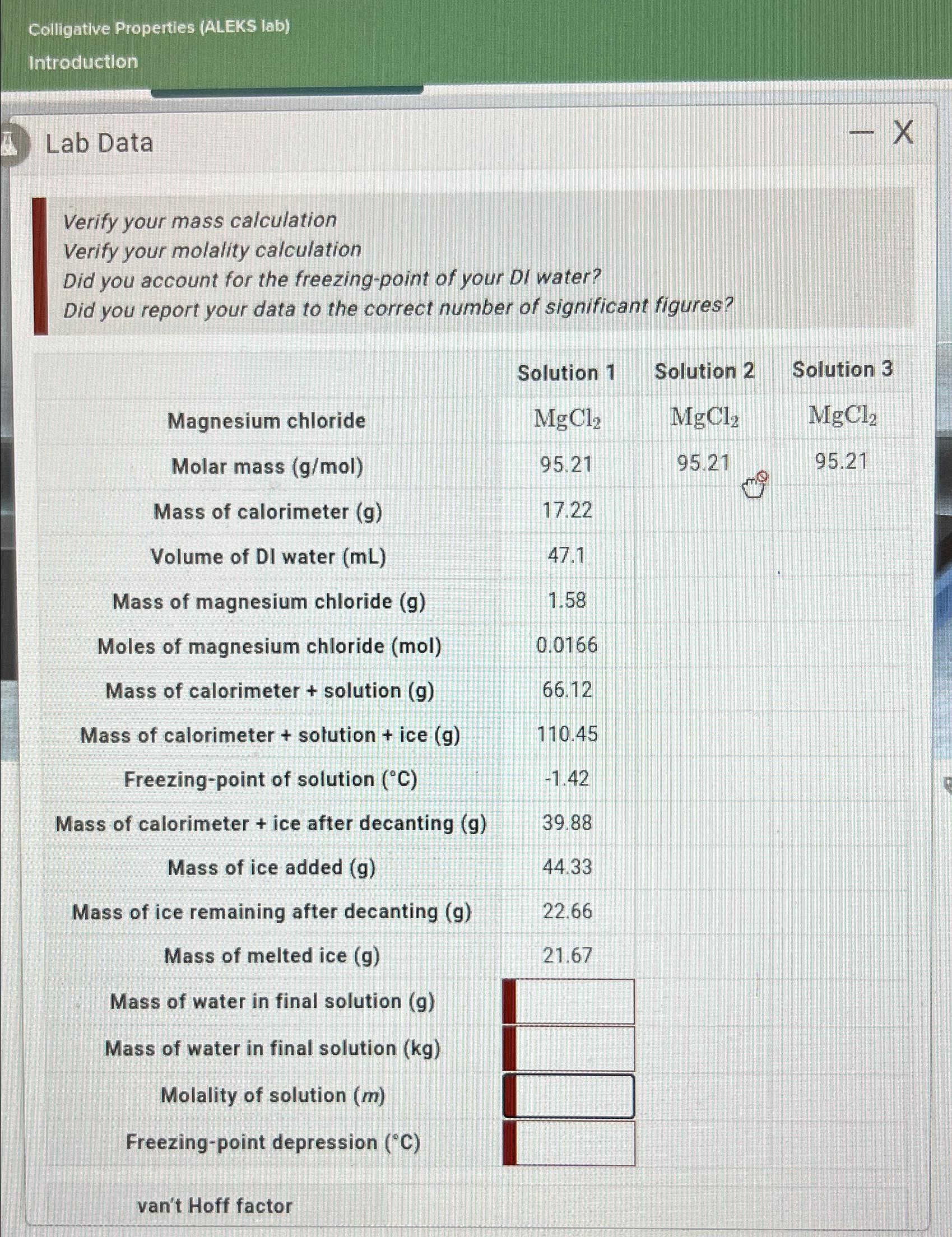
Colligative Properties (ALEKS lab) Introduction Lab Data Verify your mass calculation Verify your molality calculation Did you account for the freezing-point of your DI water? Did you report your data to the correct number of significant figures? Magnesium chloride Molar mass (g/mol) Mass of calorimeter (g) Volume of DI water (mL) Mass of magnesium chloride (g) Moles of magnesium chloride (mol) Mass of calorimeter + solution (g) Mass of calorimeter + solution + ice (g) Freezing-point of solution (C) Mass of calorimeter + ice after decanting (g) Mass of ice added (g) Mass of ice remaining after decanting (g) Mass of melted ice (g) Mass of water in final solution (g) Mass of water in final solution (kg) Molality of solution (m) Freezing-point depression (C) van't Hoff factor Solution 1 MgCl 95.21 17.22 47.1 1.58 0.0166 66.12 110.45 -1.42 39.88 44.33 22.66 21.67 Solution 2 MgC1, 95.21 - X Solution 3 MgCl 95.21
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
given that salutions males of valete Myld 00166 Weight of so... View full answer

Get step-by-step solutions from verified subject matter experts


