Compare the structure of CO and SO. Which of them has a greater dipole moment and...
Fantastic news! We've Found the answer you've been seeking!
Question:
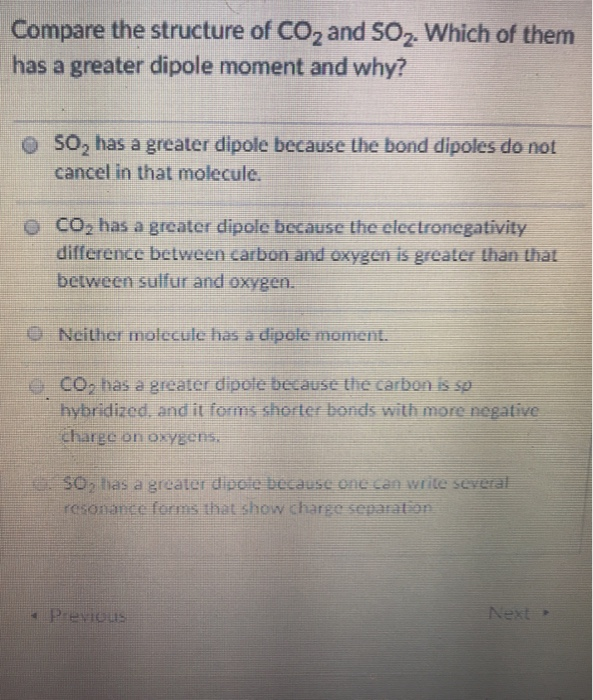
Transcribed Image Text:
Compare the structure of CO₂ and SO₂. Which of them has a greater dipole moment and why? SO₂ has a greater dipole because the bond dipoles do not cancel in that molecule. CO₂ has a greater dipole because the electronegativity difference between carbon and oxygen is greater than that between sulfur and oxygen. Neither molecule has a dipole moment. CO₂ has a greater dipole because the carbon is sp hybridized, and it forms shorter bonds with more negative charge on oxygens. SO₂ has a greater dipole because one can write several resonance forms that show charge separation Previous Next Compare the structure of CO₂ and SO₂. Which of them has a greater dipole moment and why? SO₂ has a greater dipole because the bond dipoles do not cancel in that molecule. CO₂ has a greater dipole because the electronegativity difference between carbon and oxygen is greater than that between sulfur and oxygen. Neither molecule has a dipole moment. CO₂ has a greater dipole because the carbon is sp hybridized, and it forms shorter bonds with more negative charge on oxygens. SO₂ has a greater dipole because one can write several resonance forms that show charge separation Previous Next
Expert Answer:
Answer rating: 100% (QA)
so2 has a greater dipole because the bond dipoles do not cancel in t... View the full answer

Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Posted Date:
Students also viewed these chemistry questions
-
Compare the structure of multi-level governance in these four countries; Spain, Canada, Germany, and the United Kingdom. In your paper, please discuss the structures in which countries look like each...
-
Compare the structure of the FWPCA with that of the Clean Air Act.
-
Compare the structure of cyclodecane in an all-chair conformation with that of trans-decalin. Explain why all-chair cyclodecane is highly strained, and yet trans-decalin is nearly strain free. Make...
-
You measure 30 textbooks' weights, and find they have a mean weight of 72 ounces. Assume the population standard deviation is 4 ounces. Based on this, construct a 90% confidence interval for the true...
-
Following are the unit costs of making and selling a single product at a normal level of 5,000 units per month and a current unit selling price of $90: Manufacturing costs Direct materials . $35...
-
A nozzle is required to produce a steady stream of R134a at 240 m/s at ambient conditions, 100 kPa 20C. The isentropic efficiency may be assumed to be 90%. Find by trial and error or verify...
-
Can you present a graphic that presents the payroll disbursement amounts by date for the contact employee who has been terminated but has been paid after termination (i.e., ghost employees)?
-
Ehrlich Co. began business on January 2, 2013. Salaries were paid to employees on the last day of each month, and social security tax, Medicare tax, and federal income tax were withheld in the...
-
When did 3D printing start in your selected industry? How was it first used in this industry?
-
After the success of the companys first two months, Santana Rey continues to operate Business Solutions. (Transactions for the first two months are described in the Chapter 2 serial problem.) The...
-
A bond with a maturity of 8 years and 7-year duration is priced at $1,050, and its yield to maturity is 6.94%. If the yield to maturity goes to 7.84%, you would predict that the new value of the bond...
-
1.The self-employment tax rate in US 2020 is ? 2.The maximum a 35-year-old self-employed taxpayer can contribute to his SEP is the lesser of __________ or 20% of self-employment net profit minus...
-
From 2005 to 2021, Randy worked for KY-Tech Inc. As part of his compensation package, in 2017 KY-Tech Inc. offered Randy the opportunity to purchase up to 1000 shares of KY-Tech Inc. common stock for...
-
A stock has a return of 12.9 percent and a beta of 1.27. The market return is 12.6 percent and the risk-free rate is 4.13 percent. What's the Jensen alpha of this stock?
-
"time value of money." Please provide three (3) specific examples of ways in which the time value of money impacts either specific strategic tax/financial planning decisions we have discussed or...
-
Stu is opening up a new computer service store. During November and December of 2022, he paid $20,000 in rent, $20,000 in advertising, and $20,000 in consulting. The doors to the company will open on...
-
Determine the purchase price at the indicated time before maturity of the following bond redeemed at par shown in the table below. Time Before Maturity 7 years Par Value $4,000 Bond Rate Payable...
-
Planning: Creating an Audience Profile; Collaboration: Team Projects. Compare the Facebook pages of three companies in the same industry. Analyze the content on all available tabs. What can you...
-
(a) Using Figure 19.13 as a model, sketch how the entropy of water changes as it is heated from -50C to 110C at sea level. Show the temperatures at which there are vertical increases in entropy. (b)...
-
Write the molecular and structural formulas for the compounds represented by the following models: (a) (b) (c) (d) Cl Cl
-
Little is known about the properties of astatine, At, because of its rarity and high radioactivity. Nevertheless, it is possible for us to make many predictions about its properties. (a) Do you...
-
The perfectly competitive model assumes that firms know when marginal revenue equals marginal costs. a. If a firm doesnt have this information, can it produce at the profit-maximizing level of...
-
Hundreds of music stores have been closing in the face of stagnant demand for CDs because of new competition by online music vendors. a. How would price competition from these new sources cause a...
-
A California biotechnology firm submitted a tomato that will not rot for weeks to the U.S. Food and Drug Administration. It designed such a fruit by changing the genetic structure of the tomato. What...

Study smarter with the SolutionInn App


