Question: complete answer is required asap (a) For the diffusion of carbon dioxide at atmospheric pressure and a temperature of 295 K, at what time will
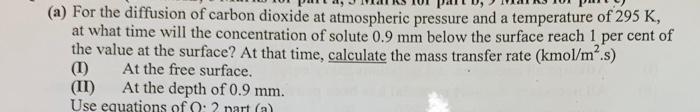
(a) For the diffusion of carbon dioxide at atmospheric pressure and a temperature of 295 K, at what time will the concentration of solute 0.9 mm below the surface reach 1 per cent of the value at the surface? At that time, calculate the mass transfer rate (kmol/m.s) At the free surface. (II) At the depth of 0.9 mm. Use equations of : 2 nart (a) (1) (a) For the diffusion of carbon dioxide at atmospheric pressure and a temperature of 295 K, at what time will the concentration of solute 0.9 mm below the surface reach 1 per cent of the value at the surface? At that time, calculate the mass transfer rate (kmol/m.s) At the free surface. (II) At the depth of 0.9 mm. Use equations of : 2 nart (a) (1)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


