Question: Complete the balanced overall ionic equation for sodium iodide dissolving in water. (wa). Nal (s) Na +1 (aq) Part 2 (1.3 points) Feedback Complete
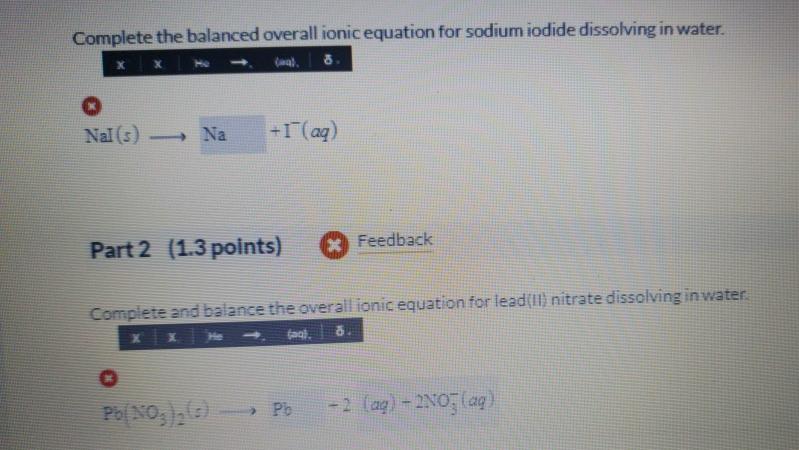
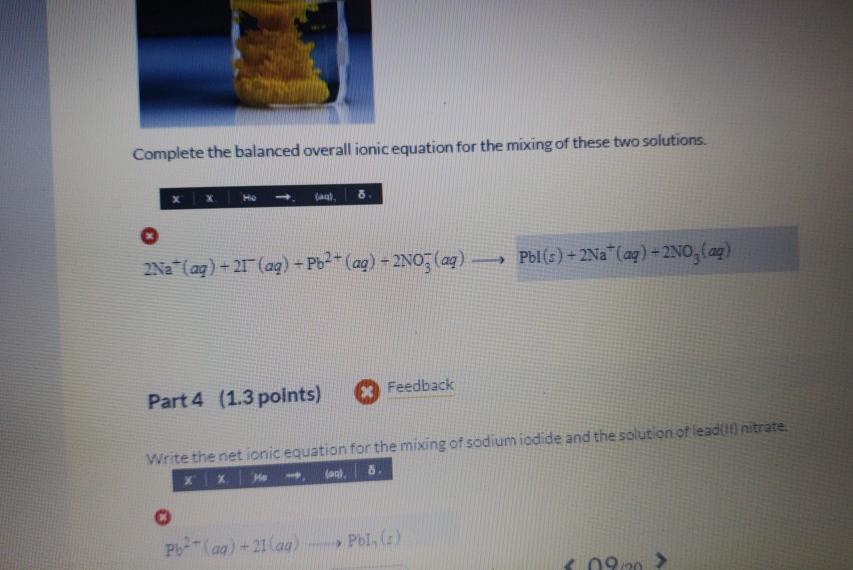
Complete the balanced overall ionic equation for sodium iodide dissolving in water. (wa). Nal (s) Na +1 (aq) Part 2 (1.3 points) Feedback Complete and balance the overall ionic equation for lead(II) nitrate dissolving in water. Pb(NO)(c)- > Pb -2 (aq)-2NO3(aq) Complete the balanced overall ionic equation for the mixing of these two solutions. x He (aq). 8. O 2Na (aq) +21 (aq) + Pb+ (aq) - 2NO3(aq) - 1 Part 4 (1.3 points) Pb+ (aq) +21(aq) Feedback Write the net ionic equation for the mixing of sodium iodide and the solution of lead(f) nitrate (a), 8. Pbl(s) + 2Na+ (aq) + 2NO(aq) -> Pbl, (z)
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


