Question: Complete the following table: 1 1 . a ) Draw the Lewis structure for ammonia ( NH 3 ) . Be sure to include any
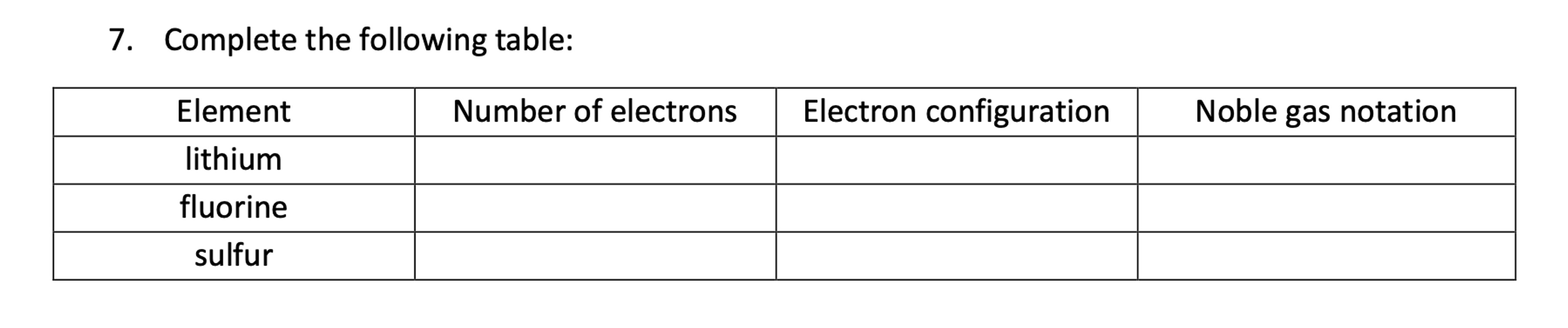
Complete the following table:
a Draw the Lewis structure for ammonia NH Be sure to include any lone pairs.
b Draw a VSEPR diagram for ammonia. What is the name for this arrangement of bonds?
c Compared to methane see question above are the bonds in ammonia closer together or
further apart? What is the reason for this?
d Between ammonia and methane, which has stronger intermolecular forces? Why is this?
e What does it mean for an ammonia molecule to form a coordinate bond? What is the result
if it forms a coordinate bond with an H ion? Hint: there is a table of polyatomic ions on page
a Draw a VSEPR diagram for acetic acid CHCOOH Identify the three different shapes ie
arrangements of bondslone pairs that are present.
b Explain why acetic acid is soluble in water. Your answer should make reference to
electronegativity differences between atoms and the shapes of the molecules.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


