Question: 1.54 Identify the formal charge and oxidation state on each atom in the following species. Assume that all valence electrons are shown. H EN H
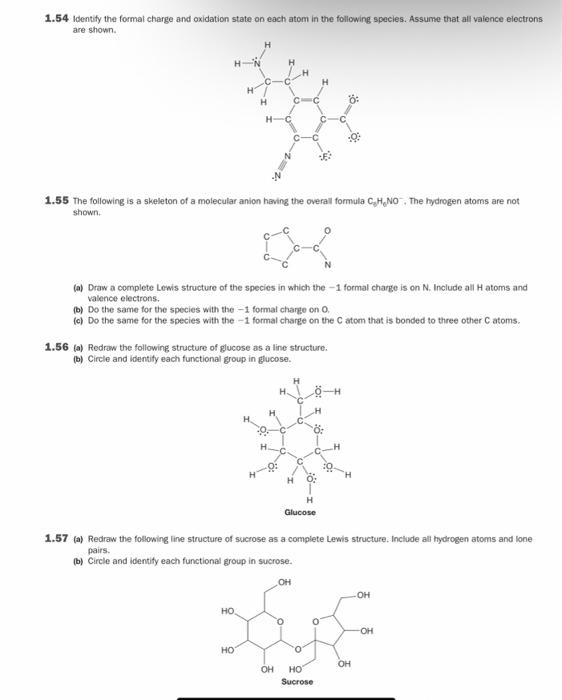
1.54 Identify the formal charge and oxidation state on each atom in the following species. Assume that all valence electrons are shown. H EN H c H H o C HC 0 C 1.55 The following is a skeleton of a molecular anion having the overall formula C.H.NO. The hydrogen atoms are not shown (a) Draw a complete Lowis structure of the species in which the 1 formal charge is on N. Include all Hatoms and valence electrons. (b) Do the same for the species with the -1 formal change on O (c) Do the same for the species with the -1 formal charge on the atom that is bonded to three other atoms. 1.56 (n) Redraw the following structure of glucose as a line structure, () Circle and Identity each functional group in glucose. H H H - Glucose 1.57 ) Redraw the following line structure of sucrose as a complete Lewis structure. Include all hydrogen atoms and lone b) Circle and identity each functional group in sucrose. pairs. OH OH HO OH HO 0 OH OH HO Sucrose
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


