Question: Complete the following table for solutions at 20 C. Solution [H3O+] (M) [OH-] (M) pH Acidic, basic, or neutral? 1 Part A Part B 10.60
![Complete the following table for solutions at 20 C. Solution [H3O+]](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f9033a57005_32166f90339f2c4c.jpg)
![(M) [OH-] (M) pH Acidic, basic, or neutral? 1 Part A Part](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f9033adde31_32266f9033a81393.jpg)
![If the pH of Solution 1 is 10.60, what is [H3O+]? Express](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f9033cba55f_32466f9033c631ca.jpg)
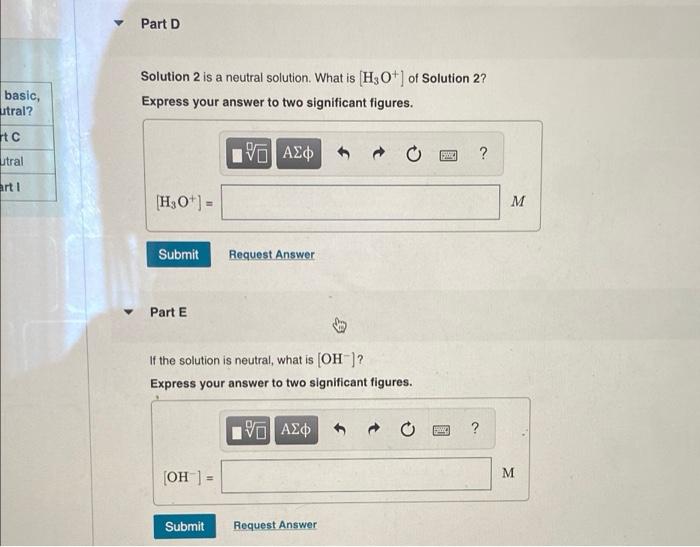
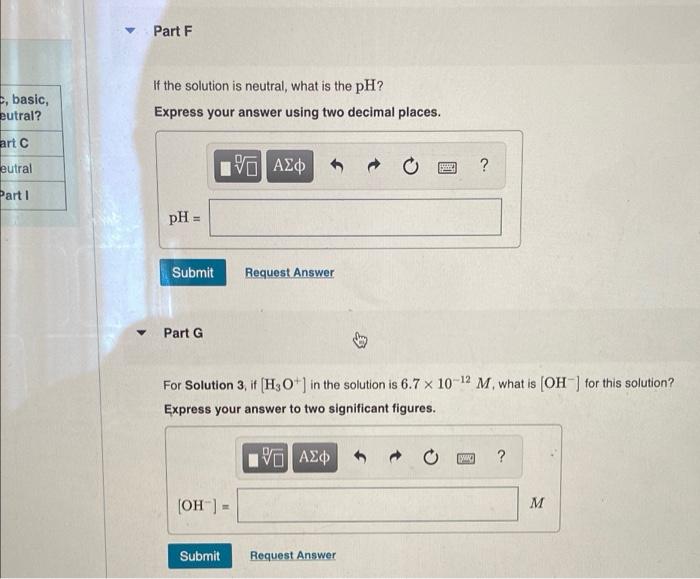
Complete the following table for solutions at 20 C. Solution [H3O+] (M) [OH-] (M) pH Acidic, basic, or neutral? 1 Part A Part B 10.60 Part C 2 Part D Part E Part F Neutral 3 6.7 x 10-12 Part G Part H Part 1 Part A If the pH of Solution 1 is 10.60, what is [H3O+]? Express your answer to two significant figures. ? H30+) = M Submit Request Answer Part B If the pH of Solution 1 is 10.60, what is (OH)? Express your answer to two significant figures. ? ? [OH) M Submit Request Answer Part D Solution 2 is a neutral solution. What is H3O+] of Solution 2? Express your answer to two significant figures. basic, utral? Htc utral 19 AEO 5 ? art 1 [H3O+] - Submit Request Answer Part E If the solution is neutral, what is (OH)? Express your answer to two significant figures. 190 AED ? M (OH) = Submit Request Answer Part F If the solution is neutral, what is the pH? Express your answer using two decimal places. , basic, eutral? art eutral Part 1 ? pH = Submit Request Answer Part G For Solution 3, if [H3O+] in the solution is 6.7 x 10-12 M. what is (OH) for this solution? Express your answer to two significant figures. ? (OH) = M Submit Request Answer Part 1 asic, al? If [H3O+] in Solution 3 is 6.7 x 10-12 M. what is the pH for this solution? Express your answer using two decimal places. al 2 | ? pH = Submit Request
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


