Question: Complete the required solution using the website (webMO) Write down the method you used This is the method, but I did not get a convincing
Complete the required solution using the website (webMO)
Write down the method you used
This is the method, but I did not get a convincing answer 
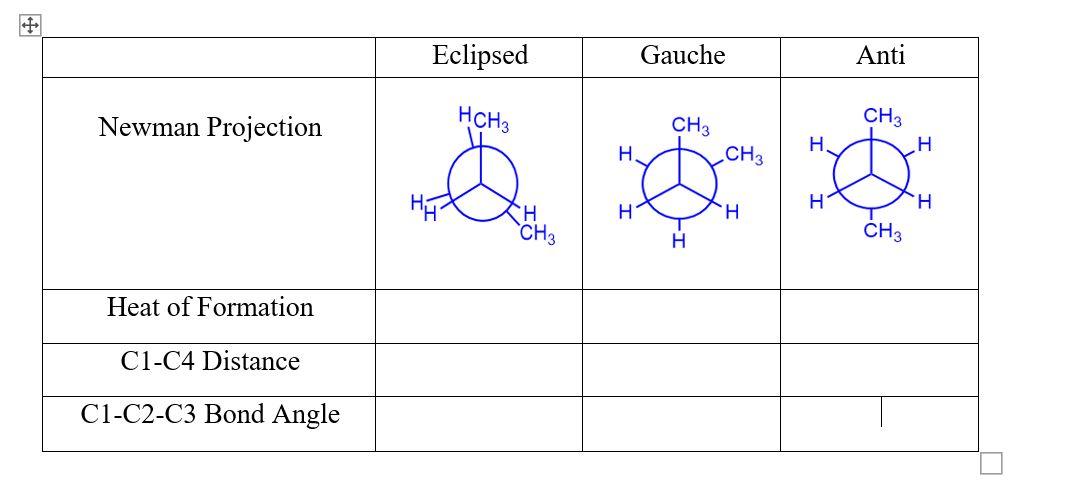
12. Return to the Job Manager, create a new job and use the editor to build butane, C4H10. Do a comprehensive cleanup using mechanics. 13. Use the Select arrow to select the four carbons. Make sure you select them linearly (i.e., C1-C2-C3-C4). From the Adjust menu choose Scan Coordinate. Set Start at 180, Stop at 180 , and \# Steps at 36 . This will allow us to create an animation sequence with 36 steps, showing the dihedral angle change from 180 to 180 degrees. 14. Close the editor and proceed to the next page. Choose the Mopac engine. Proceed to the Job Option page, and choose the Coordinate Scan calculation. Perform the calculation. This job may take 10-15 seconds. 15. Open the job and scroll down to the Coordinate Scan table. Click the magnifying glass next to the Energy column. This graph shows how energy is affected by the changing dihedral angle. Sketch it on the answer sheet. Click the filmstrip icon in the Geometry Sequence table to begin the animation. You can ontrol the animation with the five bottom buttons on the toolbar. The third button, it, is articularly useful, as it advances one frame at a time. Note how the energy (heat of ormation) changes as the molecule changes shape. Draw a Newman projection of butane in ts eclipsed, gauche, and anti conformations. Record the heat of formation at each. Find nd record the C1-C4 distance and the C1-C2-C3 bond angle for each conformer. Why is he bond angle different in the gauche and anti-conformations? \begin{tabular}{|c|c|c|c|} \hline & Eclipsed & Gauche & Anti \\ \hline Newman Projection & & & \\ \hline Heat of Formation & & & \\ \hline C1-C4 Distance & & & \\ \hline C1-C2-C3 Bond Angle & & & \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


