Question: Complete the table. That is, calculate G for the first reaction and S for the second. (Round your answer to zero decimal places.) Then, decide
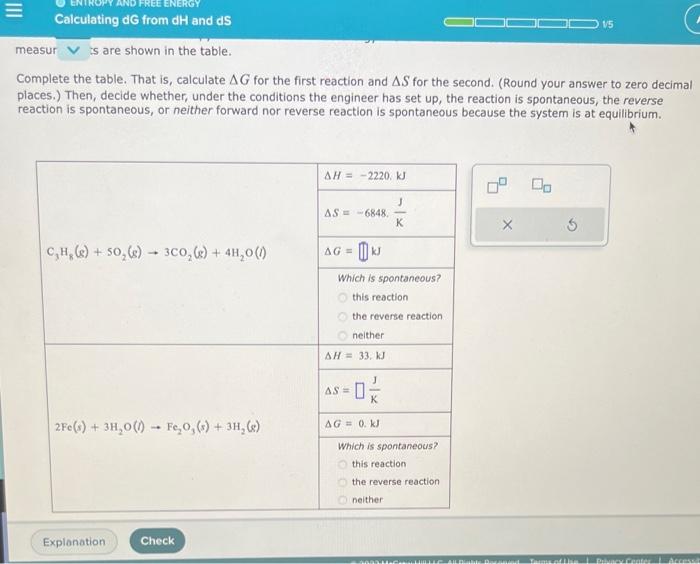
Complete the table. That is, calculate G for the first reaction and S for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


