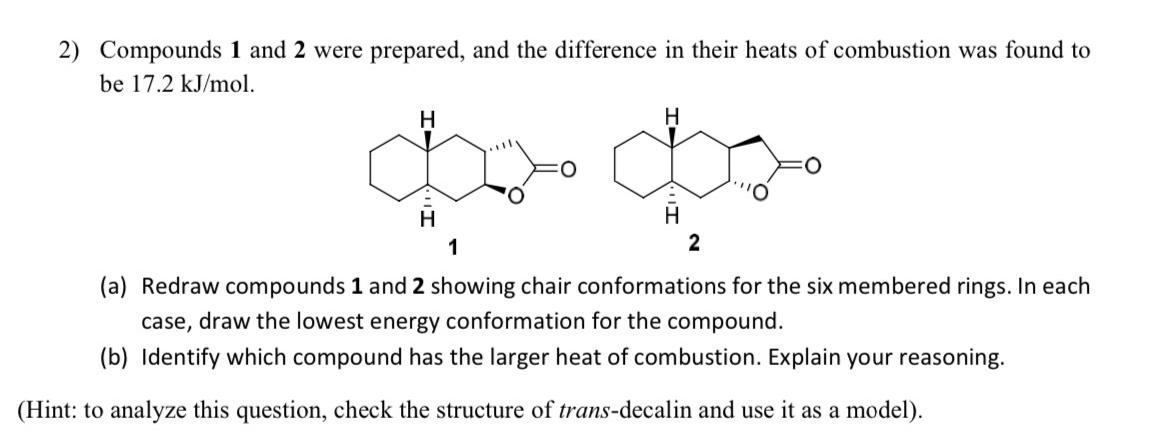
Question: Compounds 1 and 2 were prepared, and the difference in their heats of combustion was found to be 1 7 . 2 k J m
Compounds and were prepared, and the difference in their heats of combustion was found to be
a Redraw compounds and showing chair conformations for the six membered rings. In each case, draw the lowest energy conformation for the compound.
b Identify which compound has the larger heat of combustion. Explain your reasoning.
Hint: to analyze this question, check the structure of transdecalin and use it as a model
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


