Question: Concentrations as a Function of Time at 55C for the Reaction 2N2O5(g)4NO2(g)+O2(g) Use the data in the table to calculate the average rate of decomposition
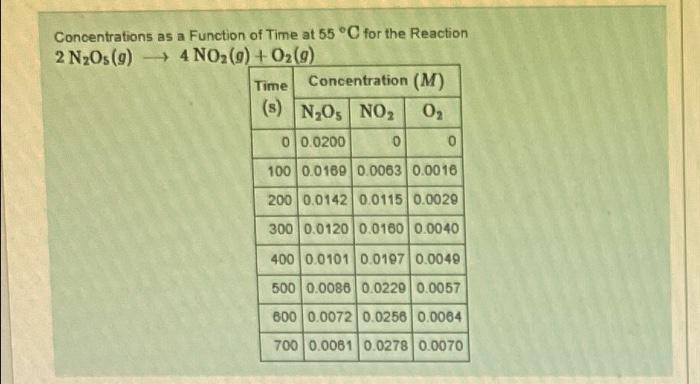
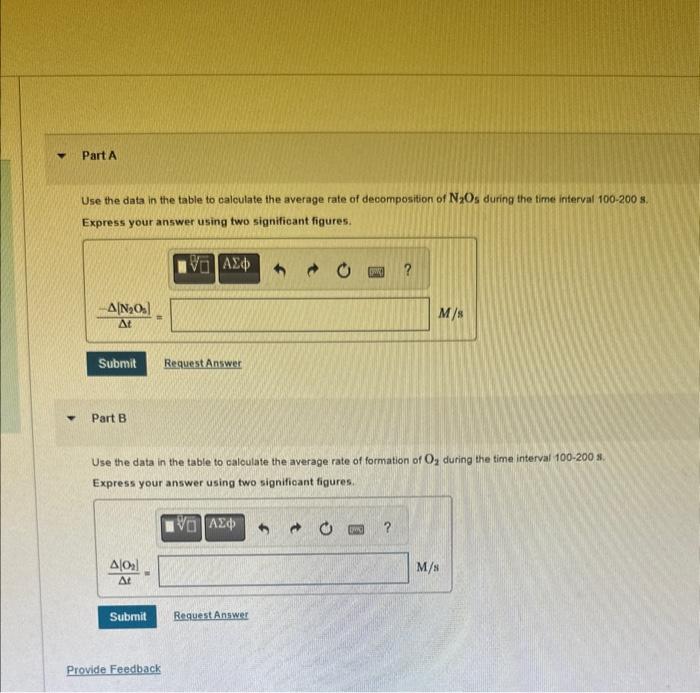
Concentrations as a Function of Time at 55C for the Reaction 2N2O5(g)4NO2(g)+O2(g) Use the data in the table to calculate the average rate of decomposition of N2O5 during the time interval 100200s. Express your answer using two significant figures. Part B Use the data in the table to caloulate the average rate of formation of O2 during the time interval 100200s. Express your answer using two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


