Question: Condenser Throttle valve Compressor 1 2 Evaporator A refrigerating system employs the vapor compression cycle, depicted above, with n-butane as the refrigerant. The following are

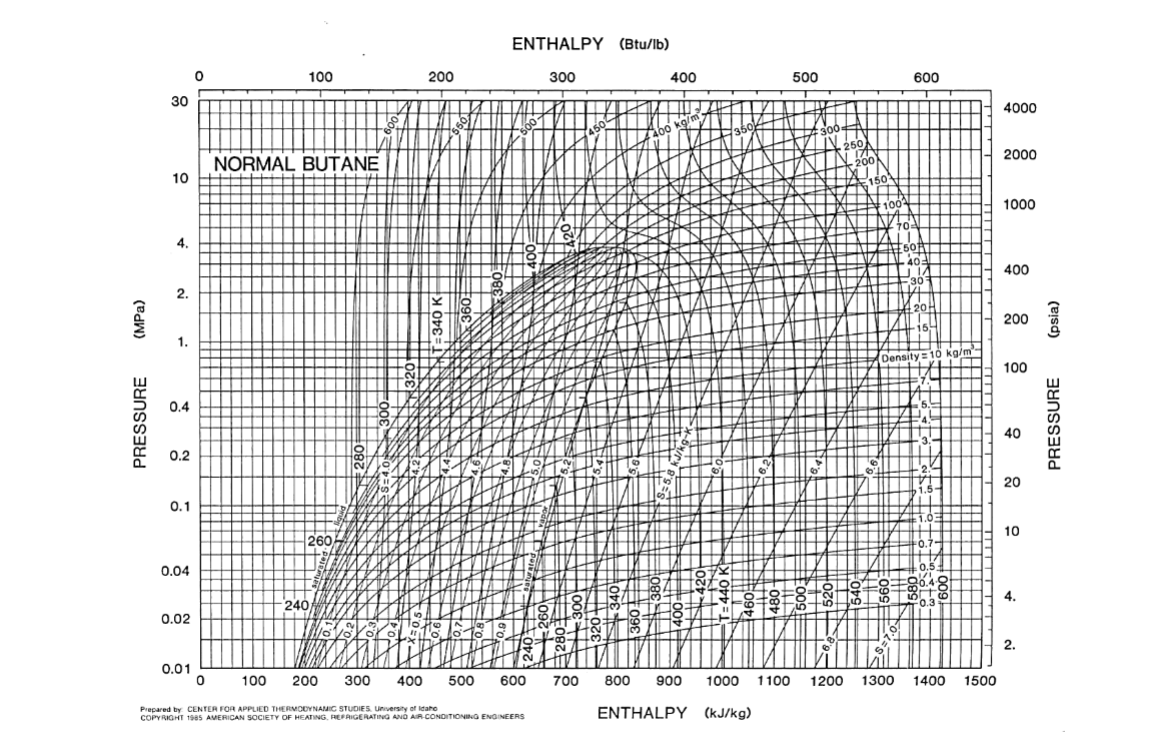
Condenser Throttle valve Compressor 1 2 Evaporator A refrigerating system employs the vapor compression cycle, depicted above, with n-butane as the refrigerant. The following are known about this system: 1. Evaporation occurs at a constant pressure of 20 kPa. The resulting saturated n-butane vapor (State 2) is then compressed to 200 kPa, and reaches the temperature of 340 K (State 3). 2. Condensation occurs at a constant pressure of 200 kPa. The resulting saturated n-butane liquid (State 4) is then throttled to 20 kPa, creating a liquid-vapor mix (State 1). (a) Mark the process of the vapor compressor cycle on the P-H diagram of n-butane, labeling the states and indicating the direction of the process. (b) Estimate the temperatures of n-butane in the evaporator and condenser, respectively. Use the Antoine Equation: 2154.7 In(psat/kPa) = 13.6608 T/K - 34.361 (c) Estimate the specific latent heat of vaporization of n-butane at the two temperatures you found in Part (b). Use the correlations of Module 14, Slide 36. (d) Calculate, without using the P-H diagram, on per kg of n-butane basis, (i) the amount of heat ejected in the condenser, (ii) the amount of heat absorbed in the evaporator, and (iii) the quality of the liquid-vapor mix entering the evaporator. You may assume that n-butane gas is ideal. Then check your answer against the P-H diagram. DATA: The critical point of n-butane is (Tc = 425.1 K, Pc = 37.96 bar), and its acentric factor is w = 0.200. The ideal-gas heat capacity of gaseous n-butane is given by: CO/R = 1.935 + 36.915 x 10-3K-1 11.402 x 10-66272 = ENTHALPY (Btu/lb) 0 100 200 300 400 500 600 30 4000 (095 600 E606 450 abo kgm 3504 300 250 171 200 2000 NORMAL BUTANE 10 150 100 1000 4. 20 501 40 | | 30 400 2. 204 (MPa) 200 (psia) 159 1. Density = 10 kg/m 100 0.4 PRESSURE PRESSURE 40 0.2 20 1.5 0.1 1.051 10 2602 0.74 0.04 0.5 4. 240 0.3 1343 , F3 0.02 57.04 2. 0.01 0 100 200 300 400 500 600 700 800 900 1000 1100 1200 1300 1400 1500 Prepared by CENTER FOR APPLIED THERMODYNAMIC STUDIES. University of Idaho COPYRIGHT 1505 AMERICAN SOCIETY OF HEATING, REFRIGERATING AND AIR CONDITIONING ENGINEERS ENTHALPY (kJ/kg)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


