Question: Conside following data on some weak acids and weak bases: acid base K K name formula name formula acetic acid HCH,CO2 1.8x10-5 pyridine CEH,N 1.7*
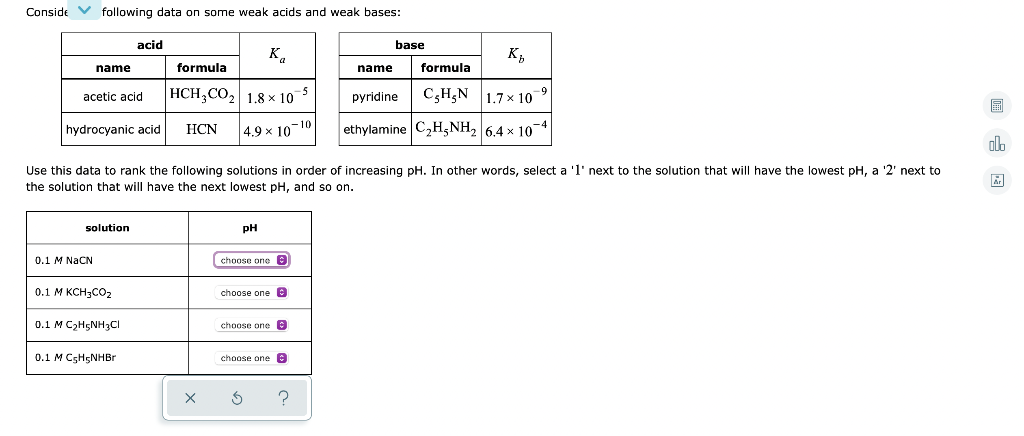
Conside following data on some weak acids and weak bases: acid base K K name formula name formula acetic acid HCH,CO2 1.8x10-5 pyridine CEH,N 1.7* 10 ethylamine C H5NH2 6,4 x 10 hydrocyanic acid HCN -4 4.9 10-10 ol. Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M NaCN choose one e 0.1 M KCH3CO2 choose one 0.1 M C H5NH2C1 choose one 0.1 M C H5NHBR choose one a 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


