Question: Consider a 100 mol mixture that is 73.0% methane (CH4) and 27.0% ethane (C2H6). To this mixture is added 30.0% excess air. Of the methane
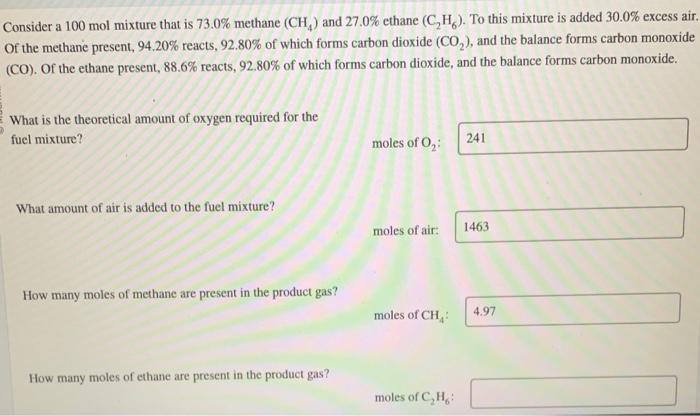
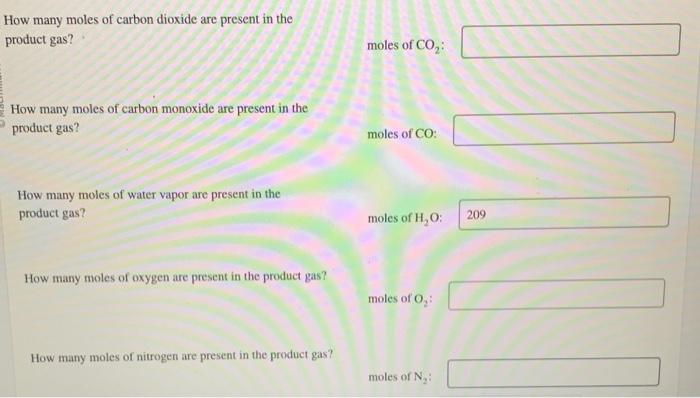
Consider a 100 mol mixture that is 73.0% methane (CH4) and 27.0% ethane (C2H6). To this mixture is added 30.0% excess air. Of the methane present, 94.20% reacts, 92.80% of which forms carbon dioxide (CO2), and the balance forms carbon monoxide (CO). Of the ethane present, 88.6% reacts, 92.80% of which forms carbon dioxide, and the balance forms carbon monoxide. What is the theoretical amount of oxygen required for the fucl mixture? molesofO2 What amount of air is added to the fuel mixture? moles of air: How many moles of methane are present in the product gas? moles of CH4 : How many moles of ethane are present in the product gas? moles of C2H6 : How many moles of carbon dioxide are present in the product gas? moles of CO2 : How many moles of carbon monoxide are present in the product gas? moles of CO: How many moles of water vapor are present in the product gas? moles of H2O How many moles of oxygen are present in the product gas? moles of O2 How many moles of nitrogen are present in the product gas? moles of N2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


