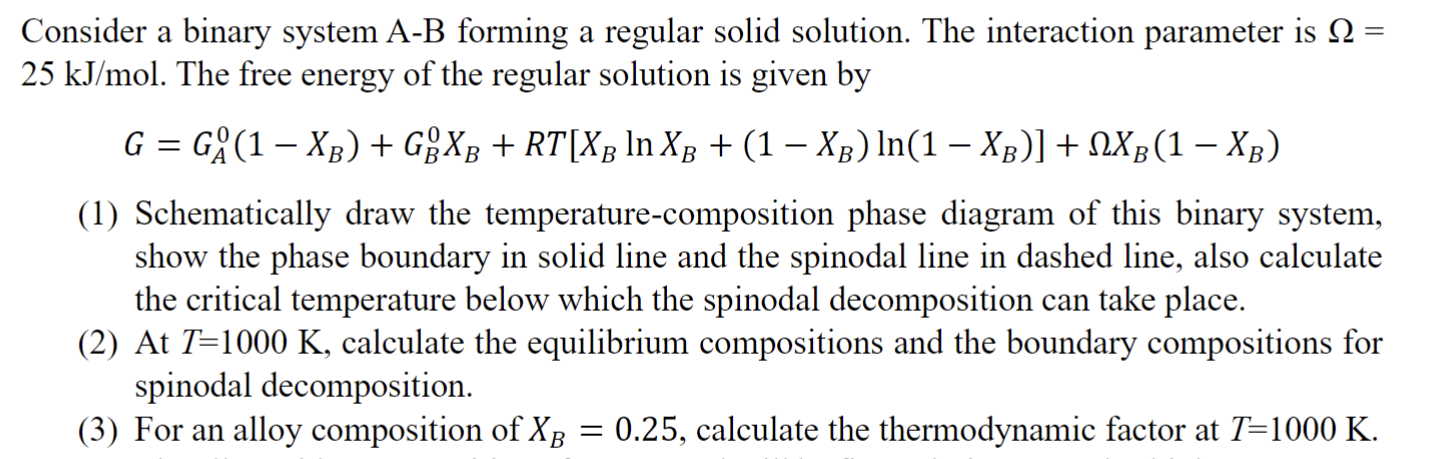
Question: Consider a binary system A - B forming a regular solid solution. The interaction parameter is = 2 5 k J m o l .
Consider a binary system AB forming a regular solid solution. The interaction parameter is
The free energy of the regular solution is given by
Schematically draw the temperaturecomposition phase diagram of this binary system,
show the phase boundary in solid line and the spinodal line in dashed line, also calculate
the critical temperature below which the spinodal decomposition can take place.
At calculate the equilibrium compositions and the boundary compositions for
spinodal decomposition.
For an alloy composition of calculate the thermodynamic factor at
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


