Question: In the A-B system, there are complete ranges of solid and liquid solutions. The liquidus and solidus show a common maximum at X A =
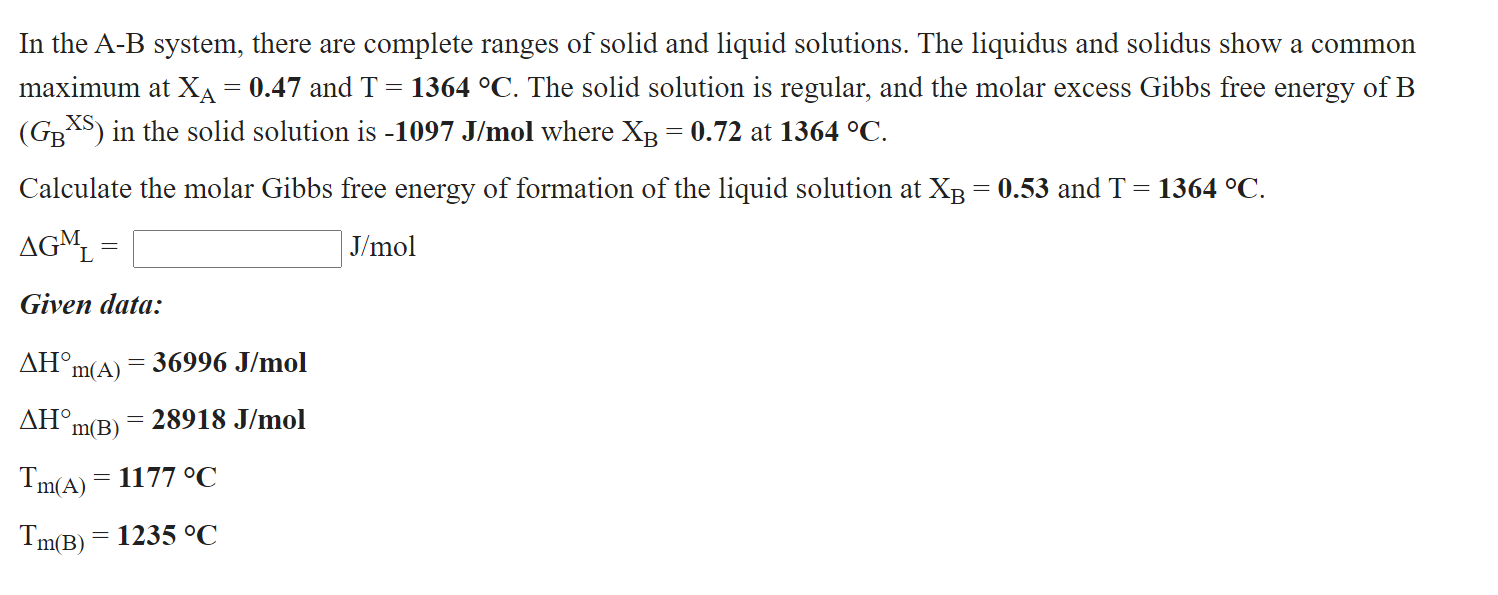
In the A-B system, there are complete ranges of solid and liquid solutions. The liquidus and solidus show a common maximum at XA = 0.47 and T = 1364 C. The solid solution is regular, and the molar excess Gibbs free energy of B (GBXS) in the solid solution is -1097 J/mol where XB = 0.72 at 1364 C.
Calculate the molar Gibbs free energy of formation of the liquid solution at XB = 0.53 and T = 1364 C.
GML = ???? J/mol
Given data:
Hm(A) = 36996 J/mol
Hm(B) = 28918 J/mol
Tm(A) = 1177 C
Tm(B) = 1235 C
In the A-B system, there are complete ranges of solid and liquid solutions. The liquidus and solidus show a common maximum at XA=0.47 and T=1364C. The solid solution is regular, and the molar excess Gibbs free energy of B (GBXS) in the solid solution is 1097J/mol where XB=0.72 at 1364C. Calculate the molar Gibbs free energy of formation of the liquid solution at XB=0.53 and T=1364C. GLM=J/mol Given data: Hm(A)=36996J/molHm(B)=28918J/molTm(A)=1177CTm(B)=1235C In the A-B system, there are complete ranges of solid and liquid solutions. The liquidus and solidus show a common maximum at XA=0.47 and T=1364C. The solid solution is regular, and the molar excess Gibbs free energy of B (GBXS) in the solid solution is 1097J/mol where XB=0.72 at 1364C. Calculate the molar Gibbs free energy of formation of the liquid solution at XB=0.53 and T=1364C. GLM=J/mol Given data: Hm(A)=36996J/molHm(B)=28918J/molTm(A)=1177CTm(B)=1235C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


